��Ŀ����
��20�֣�ij�о���ѧϰС��������롰�о�����ˮ��Ӧ���ù������ʵijɷ֡����ʼ������á�ʵ��̽��������ͬ����������⣺
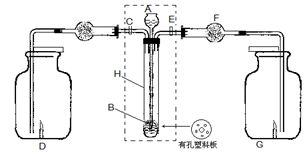
��̽��һ�������ͼ��ʾװ�ý��С�����ˮ��Ӧ����ʵ�顣
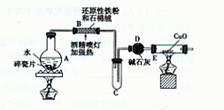
��1��Ӳ���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����ӦǰA��Ͷ�����Ƭ��Ŀ���� ��
��3��װ��E�е������� ��
��̽�������������ʵ�鷽��ȷ����Ӧ��Ӳ�ʲ������к�ɫ����ijɷ֡�
��4����Ӳ���Թ�B��ȴ��ȡ�������еĹ����������� ��������Һ�ֳ����ݡ�
��5��һ�ݵμӼ���KSCN��Һ������Һ���ɫ���ƶ�Ӳ�ʲ�����B�й������ʵijɷ֣�ѡ����ţ���ͬ�� ��������Һδ���ɫ���ƶ�Ӳ�ʲ�����B�й������ʵijɷ�
��һ����Fe3O4��������Fe ��Fe3O4��Fe ��ֻ��Fe3O4 ��ֻ��Fe
��6����һ����___ �����������ƣ����� ������֤����Һ�д���Fe2+��
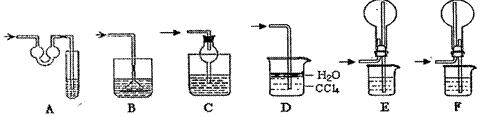
��̽���������������������������Һ��ȡ�̷����壨FeSO4��7H2O����
��Һ FeSO4��Һ
FeSO4��Һ FeSO4��7H2O����
FeSO4��7H2O����
��7������I�м���ྻ����м�������� ���û�ѧ����ʽ��ʾ����
��8������II��FeSO4ϡ��Һ�еõ�FeSO4��7H2O�������Ҫ�������� �����ȹ���____���ٹ��ˡ�Ϊ��ֹFeSO4���ʣ��������л���ע��������� ��
��̽���ġ�����������̲ⶨ��Ӧ��Ӳ�ʲ�����B�й��庬��Ԫ�ص�����������

��9���Լ�b�Ļ�ѧʽ�� ____��
��10�����㷴Ӧ��Bװ������Ԫ�ص���������Ϊ �����ݼ������жϷ�Ӧ��Ӳ���Թ�B�й������ʵijɷ��� ��
��̽��һ�������ͼ��ʾװ�ý��С�����ˮ��Ӧ����ʵ�顣

��1��Ӳ���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����ӦǰA��Ͷ�����Ƭ��Ŀ���� ��
��3��װ��E�е������� ��
��̽�������������ʵ�鷽��ȷ����Ӧ��Ӳ�ʲ������к�ɫ����ijɷ֡�
��4����Ӳ���Թ�B��ȴ��ȡ�������еĹ����������� ��������Һ�ֳ����ݡ�
��5��һ�ݵμӼ���KSCN��Һ������Һ���ɫ���ƶ�Ӳ�ʲ�����B�й������ʵijɷ֣�ѡ����ţ���ͬ�� ��������Һδ���ɫ���ƶ�Ӳ�ʲ�����B�й������ʵijɷ�
��һ����Fe3O4��������Fe ��Fe3O4��Fe ��ֻ��Fe3O4 ��ֻ��Fe
��6����һ����___ �����������ƣ����� ������֤����Һ�д���Fe2+��
��̽���������������������������Һ��ȡ�̷����壨FeSO4��7H2O����
��Һ
 FeSO4��Һ
FeSO4��Һ FeSO4��7H2O����
FeSO4��7H2O������7������I�м���ྻ����м�������� ���û�ѧ����ʽ��ʾ����
��8������II��FeSO4ϡ��Һ�еõ�FeSO4��7H2O�������Ҫ�������� �����ȹ���____���ٹ��ˡ�Ϊ��ֹFeSO4���ʣ��������л���ע��������� ��
��̽���ġ�����������̲ⶨ��Ӧ��Ӳ�ʲ�����B�й��庬��Ԫ�ص�����������

��9���Լ�b�Ļ�ѧʽ�� ____��
��10�����㷴Ӧ��Bװ������Ԫ�ص���������Ϊ �����ݼ������жϷ�Ӧ��Ӳ���Թ�B�й������ʵijɷ��� ��

��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
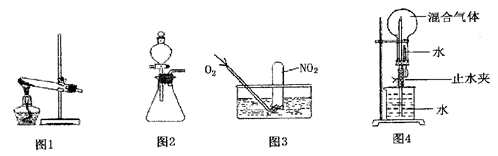


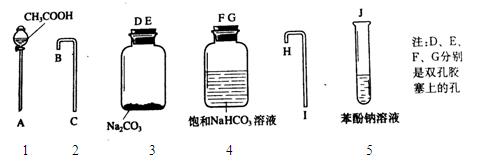
 ������һ����̽��С�������ѧ֪ʶ���������Ҵ���ˮ�����ӡ�̼�ᣨ HO��C��OH��������������ǻ�����ԭ�ӵĻ����ԡ� O
������һ����̽��С�������ѧ֪ʶ���������Ҵ���ˮ�����ӡ�̼�ᣨ HO��C��OH��������������ǻ�����ԭ�ӵĻ����ԡ� O