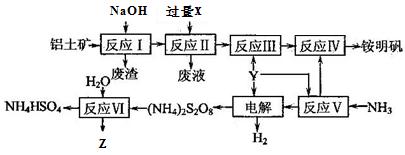
��Ŀ����
��14�֣�Br2��Fe3+ ��ˮ��ҺŨ�Ƚ�Сʱ�����ֻ�ɫ�����߶����н�ǿ�������ԡ�ij��ѧ��ȤС����ͨ��̽��ʵ�����Ƚ϶��������Ե�ǿ����
ʵ�������������������������Һ�м�������Ũ��ˮ����
ʵ��������Һ��dz��ɫ��Ϊ��ɫ��
��1��������裺
��ͬѧ��Ϊ�����ԣ�Br2 ��Fe3+ ����Ӧ�����ӷ���ʽΪ ��
��ͬѧ��Ϊ�����ԣ�Fe3+ ��Br2 ����ʹ��Һ�ʻ�ɫ�������� ���ѧʽ����
��2��ͨ����һ��ʵ����Լ�����ҵĽ���˭��˭������������Ƶ�һЩʵ�鷽����Ԥ�⣺
�ٷ���1�Ƿ���� ����ǡ�����
�ڷ���2�У����ڴ��ڷ�Ӧ �������ӷ���ʽ��ʾ����
�������ӷ���ʽ��ʾ����
����Ԥ����۲���ȷ��
�����������һ������������֤���Ѿ�������Ӧ�����й����������±���
ʵ�������������������������Һ�м�������Ũ��ˮ����
ʵ��������Һ��dz��ɫ��Ϊ��ɫ��
��1��������裺
��ͬѧ��Ϊ�����ԣ�Br2 ��Fe3+ ����Ӧ�����ӷ���ʽΪ ��
��ͬѧ��Ϊ�����ԣ�Fe3+ ��Br2 ����ʹ��Һ�ʻ�ɫ�������� ���ѧʽ����
��2��ͨ����һ��ʵ����Լ�����ҵĽ���˭��˭������������Ƶ�һЩʵ�鷽����Ԥ�⣺
| ���� | ʵ����� | Ԥ�������� |
| 1 | ȡ������ɫ��Һ������NaOH��Һ | �����ɺ��ɫ�����������ȷ |
| 2 | ȡ������ɫ��Һ���������KI��Һ | ����Һ����ɫ��������ȷ |
| 3 | ȡ������ɫ��Һ�����뱽��Һ������ | ���ϲ���Һ�ʳȺ�ɫ���� ��ȷ |
�ڷ���2�У����ڴ��ڷ�Ӧ
 �������ӷ���ʽ��ʾ����
�������ӷ���ʽ��ʾ��������Ԥ����۲���ȷ��
�����������һ������������֤���Ѿ�������Ӧ�����й����������±���
| ʵ����� | Ԥ�������� |
| | |
��14�֣���1��Br2 + 2Fe2+  2Br��+ 2Fe3+��2�֣��� Br2��2�֣���
2Br��+ 2Fe3+��2�֣��� Br2��2�֣���
��2���ң�2�֣� �ٷ�2�֣� �� 2Fe3+ + 2I�� I2 + 2Fe2+ ��2�֣�
I2 + 2Fe2+ ��2�֣�
�����������һ������������֤���Ѿ�������Ӧ��������ÿ��2�֣�
��ע������������Ҳ���֣�
 2Br��+ 2Fe3+��2�֣��� Br2��2�֣���
2Br��+ 2Fe3+��2�֣��� Br2��2�֣�����2���ң�2�֣� �ٷ�2�֣� �� 2Fe3+ + 2I��
 I2 + 2Fe2+ ��2�֣�
I2 + 2Fe2+ ��2�֣������������һ������������֤���Ѿ�������Ӧ��������ÿ��2�֣�
| ʵ����� | Ԥ�������� |
| ȡ������ɫ��Һ�����뼸��KSCN��Һ | ����Һ���ɫ����֤���Ѿ�������Ӧ |
��

��ϰ��ϵ�д�
�����Ŀ
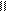 ������һ����̽��С�������ѧ֪ʶ���������Ҵ���ˮ�����ӡ�̼�ᣨ HO��C��OH��������������ǻ�����ԭ�ӵĻ����ԡ� O
������һ����̽��С�������ѧ֪ʶ���������Ҵ���ˮ�����ӡ�̼�ᣨ HO��C��OH��������������ǻ�����ԭ�ӵĻ����ԡ� O 
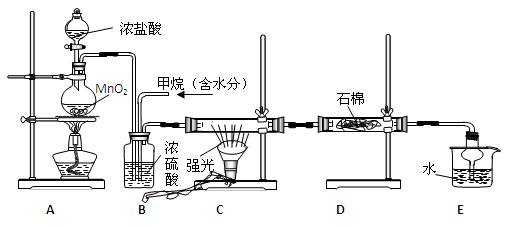

 �����ʡ�
�����ʡ�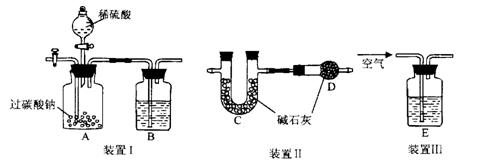
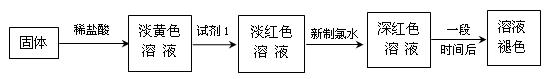
 ֤�������������壬B��ʢ��������Ba��OH��2��Һ�����۲쵽�������� ����֤���� �������ɣ�������֤��һ������ķ��� ��
֤�������������壬B��ʢ��������Ba��OH��2��Һ�����۲쵽�������� ����֤���� �������ɣ�������֤��һ������ķ��� �� װ��III��ͨ������Ŀ���� ��
װ��III��ͨ������Ŀ���� �� 2Fe+ Al2O3��ij�о�С����ʵ�������ü���װ�ý������ȷ�Ӧ���������ɵ��������ɵĺ�ɫӲ�顣С���Ա�Ʋ���Ҫԭ���Dz����������ʽ϶࣬����һ��̽���ʺ�ɫӲ�����ɡ�
2Fe+ Al2O3��ij�о�С����ʵ�������ü���װ�ý������ȷ�Ӧ���������ɵ��������ɵĺ�ɫӲ�顣С���Ա�Ʋ���Ҫԭ���Dz����������ʽ϶࣬����һ��̽���ʺ�ɫӲ�����ɡ� ����1��ȡ������ĩ���ձ��У���������3mol/LNaOH��Һ����ֽ��裬���ˣ�ϴ�ӡ�
����1��ȡ������ĩ���ձ��У���������3mol/LNaOH��Һ����ֽ��裬���ˣ�ϴ�ӡ�