ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ25Γφ ±Θ§≤ΩΖ÷Έο÷ ΒΡΒγάκΤΫΚβ≥Θ ΐ»γ±μΥυ ΨΘΚ
Μ·―ß Ϋ |
|
| HClO |
|
|
ΒγάκΤΫΚβ≥Θ ΐ |
|
|
|
|
|
Θ®1Θ©25Γφ ±Θ§Β»≈®Ε»ΒΡ![]() »ή“ΚΓΔ
»ή“ΚΓΔ![]() »ή“ΚΓΔ
»ή“ΚΓΔ![]() »ή“ΚΘ§3÷÷»ή“ΚΒΡpH”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣ________ΓΘ
»ή“ΚΘ§3÷÷»ή“ΚΒΡpH”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣ________ΓΘ
Θ®2Θ©ΙΛ“Β…œΩ…”ΟΑ±Υ°≥ΐ»ΞΈ≤Τχ![]() ΓΘΫΪ
ΓΘΫΪ![]() Ά®»κΑ±Υ°÷–Θ§Β±
Ά®»κΑ±Υ°÷–Θ§Β±![]() ΫΒ÷Ν
ΫΒ÷Ν![]() _____ΓΘ
_____ΓΘ
Θ®3Θ©≥ΘΈ¬œ¬Θ§”Ο![]() »ή“ΚΒΈΕ®
»ή“ΚΒΈΕ®![]() »ή“ΚΥυΒΟΒΈΕ®«ζœΏ»γΆΦΘΚ
»ή“ΚΥυΒΟΒΈΕ®«ζœΏ»γΆΦΘΚ
ΔΌ‘Ύ’ϊΗω Β―ιΙΐ≥Χ÷–Θ§≤Μ–η“ΣΒΡ“«ΤςΜρ”ΟΤΖ « ______Θ®Χν–ρΚ≈Θ©Θ°
![]() »ίΝΩΤΩ b ΉΕ–ΈΤΩc ΒΈΕ®ΙήΦ–d ¬©ΕΖe ≤ΘΝßΑτf ΒΈΕ®Ιή
»ίΝΩΤΩ b ΉΕ–ΈΤΩc ΒΈΕ®ΙήΦ–d ¬©ΕΖe ≤ΘΝßΑτf ΒΈΕ®Ιή
ΔΎΒΫ¥οΒΈΕ®÷’ΒψΒΡ±ξ÷Ψ « _____________ Θ°
Δέœ¬Ν–≤ΌΉςΜαΒΦ÷¬≤βΕ®ΫαΙϊΤΪΗΏΒΡ « ______ Θ°
A Φν ΫΒΈΕ®Ιή‘ΎΉΑ“Κ«ΑΈ¥”Ο±ξΉΦNaOH»ή“Κ»σœ¥
B ΒΈΕ®Ιΐ≥Χ÷–Θ§ΉΕ–ΈΤΩ“ΓΒ¥ΒΟΧΪΨγΝ“Θ§ΉΕ–ΈΤΩΡΎ”–“ΚΒΈΫΠ≥ω
C Φν ΫΒΈΕ®ΙήΦβΉλ≤ΩΖ÷‘ΎΒΈΕ®«ΑΟΜ”–Τχ≈ίΘ§ΒΈΕ®÷’Βψ ±ΖΔœ÷Τχ≈ί
D ¥οΒΫΒΈΕ®÷’Βψ ±Θ§―ω ”ΕΝ ΐ
Δή»γΆΦΒψΔΌΥυ Ψ»ή“Κ÷–![]() __________
__________![]() ΧνΓΑΘΨΓ±ΓΑΘΦΓ±ΜρΓΑ=Γ±Θ§œ¬Ά§Θ§ΒψΔΎΥυ Ψ»ή“Κ÷–ΘΚ
ΧνΓΑΘΨΓ±ΓΑΘΦΓ±ΜρΓΑ=Γ±Θ§œ¬Ά§Θ§ΒψΔΎΥυ Ψ»ή“Κ÷–ΘΚ![]() ________
________![]() Θ§ΒψΔέΥυ Ψ»ή“Κ÷–Υυ”–άκΉ”≈®Ε»”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣΘΚ_________ΓΘ
Θ§ΒψΔέΥυ Ψ»ή“Κ÷–Υυ”–άκΉ”≈®Ε»”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣΘΚ_________ΓΘ
ΓΨ¥πΑΗΓΩNa2CO3»ή“ΚΘΨNa2SO3»ή“ΚΘΨCH3COONa»ή“Κ 0.62 ade Φ”»κΉνΚσ“ΜΒΈ«β―θΜ·ΡΤΘ§»ή“Κ±δΈΣΈΔΚλ…ΪΘ§«“30sΡΎ≤ΜΆ …Ϊ AD ΘΨ ΘΦ ![]()
ΓΨΫβΈωΓΩ
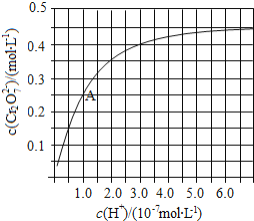
(1)ΥαΒΡΒγάκΤΫΚβ≥Θ ΐ‘Ϋ¥σΘ§ΤδΕ‘”ΠΒΡΥαΗυάκΉ”Υ°Ϋβ≥ΧΕ»‘Ϋ–ΓΘ§Β»≈®Ε»ΒΡΡΤ―Έ»ή“ΚΒΡpH‘Ϋ–ΓΘΜ
(2) ![]() =
= ![]() ΓΝ
ΓΝ![]() =
=![]() Ζ÷ΈωΦΤΥψΘΜ
Ζ÷ΈωΦΤΥψΘΜ
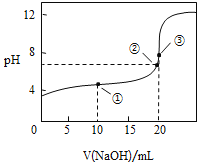
(3)ΔΌΥαΦν÷–ΚΆΒΈΕ® Β―ι÷––η“ΣΒΡ“«ΤςΈΣΥα ΫΒΈΕ®ΙήΓΔΦν ΫΒΈΕ®ΙήΓΔΒΈΕ®ΙήΦ–ΓΔΧζΦήΧ®ΓΔΫΚΆΖΒΈΙήΓΔ…’±≠ΓΔΉΕ–ΈΤΩΒ»“«ΤςΘΜΔΎNaOH»ή“Κ…‘ΙΐΝΩ ±Θ§Ζ”ΧΣ±δΈΣ«≥Κλ…ΪΘΜΔέΗυΨίc(¥ΐ≤β)= ![]() Ζ÷Έω≤ΜΒ±≤ΌΉςΕ‘V(±ξΉΦ)ΒΡ”ΑœλΘ§“‘¥Υ≈–Εœ≈®Ε»ΒΡΈσ≤νΘΜΔή”…ΆΦΩ…÷ΣΘ§ΒψΔΌΥυ Ψ»ή“ΚΈΣΒ»≈®Ε»ΒΡCH3COOHΚΆCH3COONaΒΡΜλΚœ»ή“ΚΘ§»ή“Κ≥ Υα–‘Θ§‘ρc(H+)ΘΨc(OH-)Θ§ΫαΚœ÷ Ή”ΙΊœΒΈΣc( CH3COO-)-c(CH3COOH)=2c(H+)-2c(OH-)Ζ÷ΈωΫβ¥πΘΜΒψΔΎΥυ Ψ»ή“Κ≥ ÷––‘Θ§‘ρc(H+)=c(OH-)Θ§ΗυΨίΒγΚ…ΙΊœΒΖ÷ΈωΫβ¥πΘΜΒψΔέ«ΓΚΟ…ζ≥…¥ΉΥαΡΤΘ§ΗυΨί―ΈάύΥ°ΫβΧΊΒψΖ÷Έω≈–ΕœΓΘ
Ζ÷Έω≤ΜΒ±≤ΌΉςΕ‘V(±ξΉΦ)ΒΡ”ΑœλΘ§“‘¥Υ≈–Εœ≈®Ε»ΒΡΈσ≤νΘΜΔή”…ΆΦΩ…÷ΣΘ§ΒψΔΌΥυ Ψ»ή“ΚΈΣΒ»≈®Ε»ΒΡCH3COOHΚΆCH3COONaΒΡΜλΚœ»ή“ΚΘ§»ή“Κ≥ Υα–‘Θ§‘ρc(H+)ΘΨc(OH-)Θ§ΫαΚœ÷ Ή”ΙΊœΒΈΣc( CH3COO-)-c(CH3COOH)=2c(H+)-2c(OH-)Ζ÷ΈωΫβ¥πΘΜΒψΔΎΥυ Ψ»ή“Κ≥ ÷––‘Θ§‘ρc(H+)=c(OH-)Θ§ΗυΨίΒγΚ…ΙΊœΒΖ÷ΈωΫβ¥πΘΜΒψΔέ«ΓΚΟ…ζ≥…¥ΉΥαΡΤΘ§ΗυΨί―ΈάύΥ°ΫβΧΊΒψΖ÷Έω≈–ΕœΓΘ
(1)ΥαΒΡΒγάκΤΫΚβ≥Θ ΐ‘Ϋ¥σΘ§ΤδΕ‘”ΠΒΡΥαΗυάκΉ”Υ°Ϋβ≥ΧΕ»‘Ϋ–ΓΘ§Β»≈®Ε»ΒΡΡΤ―Έ»ή“ΚΒΡpH‘Ϋ–ΓΘ§ΒγάκΤΫΚβ≥Θ ΐΘΚCH3COOHΘΨHSO3-ΘΨHCO3-Θ§‘ρΥ°Ϋβ≥ΧΕ»ΘΚCH3COO-ΘΦSO32-ΘΦCO32-Θ§Υ°Ϋβ≥ΧΕ»‘Ϋ¥σΤδœύΆ§≈®Ε»ΒΡΡΤ―Έ»ή“ΚΒΡpH‘Ϋ¥σΘ§‘ρ3÷÷»ή“ΚΒΡpH”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣΘΚNa2CO3»ή“ΚΘΨNa2SO3»ή“ΚΘΨCH3COONa»ή“ΚΘ§Ι ¥πΑΗΈΣΘΚNa2CO3»ή“ΚΘΨNa2SO3»ή“ΚΘΨCH3COONa»ή“ΚΘΜ
(2)![]() =
= ![]() ΓΝ
ΓΝ![]() =
=![]() =
=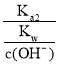 =
= =0.62Θ§Ι ¥πΑΗΈΣΘΚ0.62ΘΜ
=0.62Θ§Ι ¥πΑΗΈΣΘΚ0.62ΘΜ
(3)ΔΌΥαΦν÷–ΚΆΒΈΕ® Β―ι≤ΌΉςΘ§–η“ΣΥα ΫΒΈΕ®ΙήΓΔΦν ΫΒΈΕ®ΙήΓΔΒΈΕ®ΙήΦ–ΓΔΧζΦήΧ®ΓΔ…’±≠ΓΔΉΕ–ΈΤΩΒ»“«ΤςΘ§≤Μ–η“Σ»ίΝΩΤΩΓΔ¬©ΕΖΓΔ≤ΘΝßΑτΘ§Ι ¥πΑΗΈΣΘΚadeΘΜ
ΔΎNaOH»ή“Κ…‘ΙΐΝΩ ±Θ§Ζ”ΧΣ±δΈΣ«≥Κλ…ΪΘ§Υυ“‘NaOH»ή“ΚΒΈΕ®CH3COOH¥οΒΫΒΈΕ®÷’Βψ ±Θ§Φ”»κΉνΚσ“ΜΒΈNaOH»ή“ΚΘ§Ζ”ΧΣ±δΈΣ«≥Κλ…ΪΘ§≤Δ«“30sΡΎ≤ΜΆ …ΪΘ§
Ι ¥πΑΗΈΣΘΚΦ”»κΉνΚσ“ΜΒΈ«β―θΜ·ΡΤΘ§»ή“Κ±δΈΣ«≥Κλ…ΪΘ§«“30sΡΎ≤ΜΆ …ΪΘΜ
ΔέAΘ°Φν ΫΒΈΕ®Ιή‘ΎΉΑ“Κ«ΑΈ¥”Ο±ξΉΦNaOH»ή“Κ»σœ¥Θ§ΒΦ÷¬œϊΚΡ±ξΉΦ“ΚΧεΜΐΤΪΗΏΘ§ΗυΨίc(¥ΐ≤β)= ![]() Ζ÷ΈωΩ…÷ΣΘ§¥ΐ≤β“Κ≈®Ε»ΤΪΗΏΘ§Ι A―ΓΘΜBΘ°ΒΈΕ®Ιΐ≥Χ÷–Θ§ΉΕ–ΈΤΩ“ΓΒ¥ΒΟΧΪΨγΝ“Θ§ΉΕ–ΈΤΩΡΎ”–“ΚΒΈΫΠ≥ωΘ§ΒΦ÷¬œϊΚΡ±ξΉΦ“ΚΧεΜΐΤΪΒΆΘ§ΗυΨίc(¥ΐ≤β)=
Ζ÷ΈωΩ…÷ΣΘ§¥ΐ≤β“Κ≈®Ε»ΤΪΗΏΘ§Ι A―ΓΘΜBΘ°ΒΈΕ®Ιΐ≥Χ÷–Θ§ΉΕ–ΈΤΩ“ΓΒ¥ΒΟΧΪΨγΝ“Θ§ΉΕ–ΈΤΩΡΎ”–“ΚΒΈΫΠ≥ωΘ§ΒΦ÷¬œϊΚΡ±ξΉΦ“ΚΧεΜΐΤΪΒΆΘ§ΗυΨίc(¥ΐ≤β)= ![]() Ζ÷ΈωΩ…÷ΣΘ§¥ΐ≤β“Κ≈®Ε»ΤΪΒΆΘ§Ι B≤Μ―ΓΘΜCΘ°Φν ΫΒΈΕ®ΙήΦβΉλ≤ΩΖ÷‘ΎΒΈΕ®«ΑΟΜ”–Τχ≈ίΘ§ΒΈΕ®÷’Βψ ±ΖΔœ÷Τχ≈ίΘ§ΒΦ÷¬œϊΚΡ±ξΉΦ“ΚΧεΜΐΕΝ ΐΤΪΒΆΘ§ΗυΨίc(¥ΐ≤β)=
Ζ÷ΈωΩ…÷ΣΘ§¥ΐ≤β“Κ≈®Ε»ΤΪΒΆΘ§Ι B≤Μ―ΓΘΜCΘ°Φν ΫΒΈΕ®ΙήΦβΉλ≤ΩΖ÷‘ΎΒΈΕ®«ΑΟΜ”–Τχ≈ίΘ§ΒΈΕ®÷’Βψ ±ΖΔœ÷Τχ≈ίΘ§ΒΦ÷¬œϊΚΡ±ξΉΦ“ΚΧεΜΐΕΝ ΐΤΪΒΆΘ§ΗυΨίc(¥ΐ≤β)= ![]() Ζ÷ΈωΩ…÷ΣΘ§¥ΐ≤β“Κ≈®Ε»ΤΪΒΆΘ§Ι C≤Μ―ΓΘΜDΘ°¥οΒΫΒΈΕ®÷’Βψ ±Θ§―ω ”ΕΝ ΐΘ§ΒΦ÷¬œϊΚΡ±ξΉΦ“ΚΧεΜΐΕΝ ΐΤΪΗΏΘ§ΗυΨίc(¥ΐ≤β)=
Ζ÷ΈωΩ…÷ΣΘ§¥ΐ≤β“Κ≈®Ε»ΤΪΒΆΘ§Ι C≤Μ―ΓΘΜDΘ°¥οΒΫΒΈΕ®÷’Βψ ±Θ§―ω ”ΕΝ ΐΘ§ΒΦ÷¬œϊΚΡ±ξΉΦ“ΚΧεΜΐΕΝ ΐΤΪΗΏΘ§ΗυΨίc(¥ΐ≤β)= ![]() Ζ÷ΈωΩ…÷ΣΘ§¥ΐ≤β“Κ≈®Ε»ΤΪΗΏΘ§Ι D―ΓΘΜΙ ¥πΑΗΈΣΘΚADΘΜ
Ζ÷ΈωΩ…÷ΣΘ§¥ΐ≤β“Κ≈®Ε»ΤΪΗΏΘ§Ι D―ΓΘΜΙ ¥πΑΗΈΣΘΚADΘΜ
Δή”…ΆΦΩ…÷ΣΘ§ΒψΔΌΥυ Ψ»ή“ΚΈΣΒ»≈®Ε»ΒΡCH3COOHΚΆCH3COONaΒΡΜλΚœ»ή“ΚΘ§»ή“Κ≥ Υα–‘Θ§‘ρc(H+)ΘΨc(OH-)Θ§¥Υ ±÷ Ή”ΙΊœΒΈΣc( CH3COO-)-c(CH3COOH)=2c(H+)-2c(OH-)Θ§Φ¥c( CH3COO-)+c(OH-)=c(CH3COOH)+c(H+)+[(H+)-c(OH-)]ΘΨc(CH3COOH)+c(H+)ΘΜΒψΔΎΥυ Ψ»ή“Κ≥ ÷––‘Θ§‘ρc(H+)=c(OH-)Θ§ΒγΚ…ΙΊœΒΈΣc( CH3COO-)+c(OH-)=c(Na+)+c(H+)Θ§Φ¥c(Na+)=c( CH3COO-)Θ§ΒΪ»ή“Κ÷–ΜΙ”–ΈΔΝΩCH3COOHΘ§Υυ“‘c(Na+)ΘΦc( CH3COO-)+c(CH3COOH)ΘΜΒψΔέ«ΓΚΟ…ζ≥…¥ΉΥαΡΤΘ§ΗυΨί―ΈάύΥ°ΫβΧΊΒψΩ…÷ΣΘΚ![]() ΘΜΙ ¥πΑΗΈΣΘΚΘΨΘΜΘΦΘΜ
ΘΜΙ ¥πΑΗΈΣΘΚΘΨΘΜΘΦΘΜ![]() ΓΘ
ΓΘ