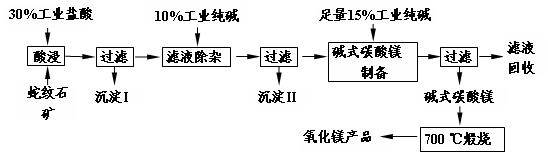
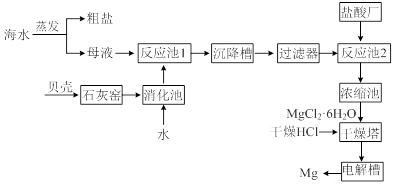
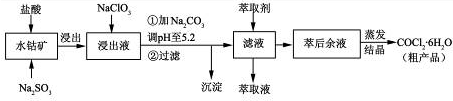
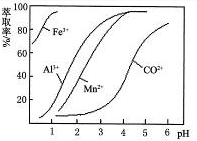
��Ŀ����
��15�֣��ظ����ƣ�Na2Cr2O7����Ҫ����ӡȾ���Ƹҽҩ����Ƶȡ���ҵ���Ը�������Ҫ�ɷ�FeO��Cr2O3����̼���ơ�����������Ϊԭ�������ظ����ƣ�Na2Cr2O7��2H2O������Ҫ��Ӧ���£�
��4FeO��Cr2O3+8Na2CO3+7O2 8Na2CrO4+2Fe2O3+8CO2
8Na2CrO4+2Fe2O3+8CO2
��2Na2Cr04+H2S04��-----~Na2S04+Na2Cr207+H20
��1����Ӧ�����ڻ�תҤ�н��У���Ӧʱ�費�Ͻ��裬
��Ŀ���� ��
��
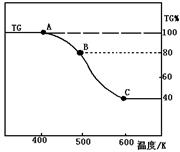
��2����ͼ�Ǻ췯�ƣ�Na2Cr2O7��2H2O����Na2SO4����

������ߡ���Na2Cr2O7��Na2SO4�Ļ����Һ����ȡNa2Cr2O7��
����IJ������Ƚ������Һ����Ũ�������ȹ��ˡ����ȹ��˵�Ŀ���� ��Ȼ����Һ ���Ӷ������췯�ơ�
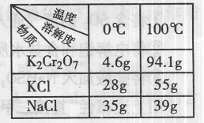
��3��Na2Cr2O7��KCl��K2SO4���и��ֽⷴӦ����ȡK2Cr2O7����
��Na2Cr2O7��KCl���Ʊ�K2Cr2O7�������������裨�й����ʵ���
��ȼ��ұ����� ��
��4���ظ���ؿ���������ʯ�����ĺ����ⶨ��ʵ�鲽�����£�
����1����mg����ʯ��Ũ��������� ��
��
����2������SnCl2��Һ��Fe3+��ԭ����ɫ��ʧ
����3����������Һ��ȴ������HgC2��Һ����������Sn2+����
ΪSn4+
����4������15mL���������Ļ���ἰ5��O��2��������������ָʾ��
����5��������cmol��L���ظ������Һ�ζ�����Һ���ȶ���ɫ����Ϊ�յ㣬�����ظ������ҺVmL
��д��SnCl2��ԭFe3+�����ӷ���ʽ ��
����ʡȥ����ۣ������ⶨ�����ĺ��� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
�۲���5ʹ�õIJ���������
��4FeO��Cr2O3+8Na2CO3+7O2
 8Na2CrO4+2Fe2O3+8CO2
8Na2CrO4+2Fe2O3+8CO2��2Na2Cr04+H2S04��-----~Na2S04+Na2Cr207+H20
��1����Ӧ�����ڻ�תҤ�н��У���Ӧʱ�費�Ͻ��裬
��Ŀ����
 ��
����2����ͼ�Ǻ췯�ƣ�Na2Cr2O7��2H2O����Na2SO4����

������ߡ���Na2Cr2O7��Na2SO4�Ļ����Һ����ȡNa2Cr2O7��
����IJ������Ƚ������Һ����Ũ�������ȹ��ˡ����ȹ��˵�Ŀ���� ��Ȼ����Һ ���Ӷ������췯�ơ�
��3��Na2Cr2O7��KCl��K2SO4���и��ֽⷴӦ����ȡK2Cr2O7����
��Na2Cr2O7��KCl���Ʊ�K2Cr2O7�������������裨�й����ʵ���
��ȼ��ұ����� ��

��4���ظ���ؿ���������ʯ�����ĺ����ⶨ��ʵ�鲽�����£�
����1����mg����ʯ��Ũ���������
 ��
������2������SnCl2��Һ��Fe3+��ԭ����ɫ��ʧ
����3����������Һ��ȴ������HgC2��Һ����������Sn2+����
ΪSn4+
����4������15mL���������Ļ���ἰ5��O��2��������������ָʾ��
����5��������cmol��L���ظ������Һ�ζ�����Һ���ȶ���ɫ����Ϊ�յ㣬�����ظ������ҺVmL
��д��SnCl2��ԭFe3+�����ӷ���ʽ ��
����ʡȥ����ۣ������ⶨ�����ĺ��� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
�۲���5ʹ�õIJ���������

��

��ϰ��ϵ�д�
 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
�����Ŀ