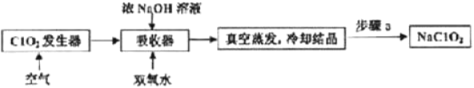
ЬтФПФкШн
ЁОЬтФПЁПNH3ЁЂNOxЁЂSO2ДІРэВЛЕБвздьГЩЛЗОГЮлШОЃЌШчЙћЖдетаЉЦјЬхМгвдРћгУОЭПЩвдБфЗЯЮЊБІЃЌМШМѕЩйСЫЖдЛЗОГЕФЮлШОЃЌгжНтОіСЫВПЗжФмдДЮЃЛњЮЪЬтЁЃ
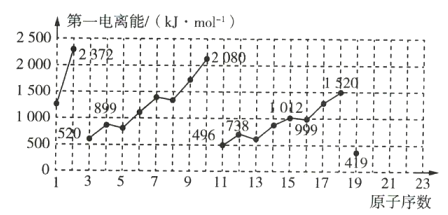
ЃЈlЃЉЯѕЫсГЇГЃгУДпЛЏЛЙдЗНЗЈДІРэЮВЦјЁЃCH4дкДпЛЏЬѕМўЯТПЩвдНЋNO2ЛЙдЮЊN2ЁЃвбжЊЃК
![]() Ђй
Ђй
![]() Ђк
Ђк
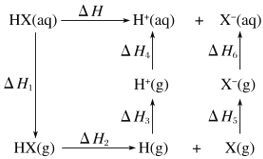
дђЗДгІ![]() ЃЈ1ЃЉЁїH=_______
ЃЈ1ЃЉЁїH=_______
ЃЈ2ЃЉЙЄвЕЩЯРћгУАБЦјЩњВњЧтЧшЫсЃЈHCNЃЉЕФЗДгІЮЊЃК
ЂйдквЛЖЈЮТЖШЬѕМўЯТЃЌЯђ2LКуШнУмБеШнЦїжаМгШы2 mol CH4КЭ2 mol NH3ЃЌЦНКтЪБNH3ЬхЛ§ЗжЪ§ЮЊ30%ЃЌЫљгУЪБМфЮЊ10 minЃЌдђИУЪБМфЖЮФкгУCH4ЕФХЈЖШБфЛЏБэЪОЕФЗДгІЫйТЪЮЊ______ molЁЄL-lЁЄmin-1ЃЌИУЮТЖШЯТЦНКтГЃЪ§K= ___ЁЃШєБЃГжЮТЖШВЛБфЃЌдйЯђШнЦїжаМгШыCH4КЭH2Иї1 molЃЌдђДЫЪБvе§ ___ЃЈЬюЁА>ЁБЁА=ЁБЛђЁА<ЁБЃЉvФц ЁЃ
ЂкЦфЫћЬѕМўвЛЖЈЃЌДяЕНЦНКтЪБNH3зЊЛЏТЪЫцЭтНчЬѕМўXБфЛЏЕФЙиЯЕШчЭМ1ЫљЪОЁЃXДњБэ ___ЃЈЬюзжФИДњКХЃЉЁЃ
A ЮТЖШ B бЙЧП C дСЯжаCH4гыNH3ЕФЬхЛ§БШ
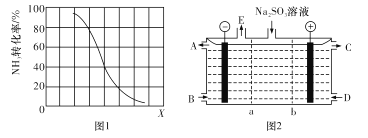
ЃЈ3ЃЉФГбаОПаЁзщгУNaOHШмвКЮќЪеЮВЦјжаЕФЖўбѕЛЏСђЃЌНЋЕУЕНЕФNa2SO3НјааЕчНтЩњВњСђЫсЃЌЦфжавѕЁЂбєФЄзщКЯЕчНтзАжУШчЭМ2ЫљЪОЃЌЕчМЋВФСЯЮЊЪЏФЋЁЃA--EЗжБ№ДњБэЩњВњжаЕФдСЯЛђВњЦЗЃЌbБэЪО____ЃЈЬюЁАвѕЁБЛђЁАбєЁБЃЉРызгНЛЛЛФЄЁЃбєМЋЕФЕчМЋЗДгІЪНЮЊ_______
ЁОД№АИЁП-958.0kJmol-1 0.025 0.1875(mol/L)2 ЃМ B вѕ SO32--2e-+H2O=2H++ SO42-
ЁОНтЮіЁП
(1)ИљОнИЧЫЙЖЈТЩЗжЮіМЦЫуЃЛ
(2)ЂйИљОнШ§ЖЮЪННсКЯЦНКтЪБNH3ЬхЛ§ЗжЪ§ЮЊ30%МЦЫуГіЗДгІЕФАБЦјЕФЮяжЪЕФСПЃЌдйНсКЯv(CH4)=![]() КЭK=
КЭK=![]() МЦЫуЃЌБЃГжЮТЖШВЛБфЃЌдйЯђШнЦїжаМгШыCH4КЭH2Иї1 molЃЌИљОнQcгыKЕФЙиЯЕХаЖЯЃЛ
МЦЫуЃЌБЃГжЮТЖШВЛБфЃЌдйЯђШнЦїжаМгШыCH4КЭH2Иї1 molЃЌИљОнQcгыKЕФЙиЯЕХаЖЯЃЛ
ЂкИљОнЭМЪОЃЌXдНДѓЃЌзЊЛЏТЪдНаЁЃЌЦНКтФцЯђвЦЖЏЃЌНсКЯЦНКтвЦЖЏЕФгАЯьвђЫиЗжЮіХаЖЯЃЛ
(3) гЩЕУЕНЕФNa2SO3НјааЕчНтЩњВњСђЫсЃЌСђЫсИљРДдДгкбЧСђЫсИљЕФЗХЕчЃЌИУЙ§ГЬЗЂЩњбѕЛЏЗДгІЃЌдкбєМЋЗЂЩњЃЌОнДЫЗжЮіНтД№ЁЃ
(1) Ђй![]() ЃЌЂк
ЃЌЂк![]() ЃЌCH4дкДпЛЏЬѕМўЯТПЩвдНЋNO2ЛЙдЮЊN2ЗЂЩњЕФЗДгІЮЊЃКCH4(g)+2NO2(g)=CO2(g)+N2(g)+2H2O(g)ЃЌИљОнИЧЫЙЖЈТЩПЩжЊЃЌЂй-ЂкПЩЕУШШЛЏбЇЗНГЬЪНЃКCH4(g)+2NO2(g)=CO2(g)+N2(g)+2H2O(g)ЁїH=(-890.3kJmol-1)-(+67.7kJmol-1)=-958.0kJmol-1ЃЌЙЪД№АИЮЊЃК -958.0kJmol-1ЃЛ
ЃЌCH4дкДпЛЏЬѕМўЯТПЩвдНЋNO2ЛЙдЮЊN2ЗЂЩњЕФЗДгІЮЊЃКCH4(g)+2NO2(g)=CO2(g)+N2(g)+2H2O(g)ЃЌИљОнИЧЫЙЖЈТЩПЩжЊЃЌЂй-ЂкПЩЕУШШЛЏбЇЗНГЬЪНЃКCH4(g)+2NO2(g)=CO2(g)+N2(g)+2H2O(g)ЁїH=(-890.3kJmol-1)-(+67.7kJmol-1)=-958.0kJmol-1ЃЌЙЪД№АИЮЊЃК -958.0kJmol-1ЃЛ
(2)ЂйЩш10 minЪБЃЌЗДгІЕФАБЦјЕФЮяжЪЕФСПЮЊxЃЌ
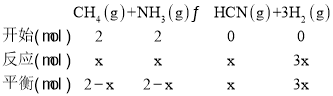
дђ![]() ЁС100%=30%ЃЌНтЕУЃКx=0.5ЃЌвђДЫv(CH4)=
ЁС100%=30%ЃЌНтЕУЃКx=0.5ЃЌвђДЫv(CH4)=![]() =
=![]() =0.025 molЁЄL-lЁЄmin-1ЃЌИУЮТЖШЯТЃЌK=
=0.025 molЁЄL-lЁЄmin-1ЃЌИУЮТЖШЯТЃЌK=![]() =
=![]() =0.1875(mol/L)2ЃЌБЃГжЮТЖШВЛБфЃЌдйЯђШнЦїжаМгШыCH4КЭH2Иї1 molЃЌДЫЪБQc=
=0.1875(mol/L)2ЃЌБЃГжЮТЖШВЛБфЃЌдйЯђШнЦїжаМгШыCH4КЭH2Иї1 molЃЌДЫЪБQc=![]() =
=![]() =0.5208(mol/L)2ЃОKЃЌЦНКтФцЯђвЦЖЏЃЌдђvе§ ЃМvФцЃЌЙЪД№АИЮЊЃК0.025ЃЛ0.1875(mol/L)2ЃЛЃМЃЛ
=0.5208(mol/L)2ЃОKЃЌЦНКтФцЯђвЦЖЏЃЌдђvе§ ЃМvФцЃЌЙЪД№АИЮЊЃК0.025ЃЛ0.1875(mol/L)2ЃЛЃМЃЛ
ЂкИљОнЭМЪОЃЌXдНДѓЃЌзЊЛЏТЪдНаЁЃЌЦНКтФцЯђвЦЖЏЁЃИУЗДгІЮЊЮќШШЗДгІЃЌЮТЖШЩ§ИпЃЌЦНКте§ЯђвЦЖЏЃЌзЊЛЏТЪдіДѓЃЌДэЮѓЃЛдіДѓбЙЧПЃЌЦНКтФцЯђвЦЖЏЃЌзЊЛЏТЪМѕаЁЃЌе§ШЗЃЛдСЯжаCH4ЕФЬхЛ§дНДѓЃЌАБЦјЕФзЊЛЏТЪдНИпЃЌДэЮѓЃЛгыЭМЯѓЗћКЯЕФЪЧBЃЌЙЪД№АИЮЊЃКBЃЛ
(3) гЩЕУЕНЕФNa2SO3НјааЕчНтЩњВњСђЫсЃЌСђЫсИљРДдДгкбЧСђЫсИљЕФЗХЕчЃЌИУЙ§ГЬЗЂЩњбѕЛЏЗДгІЃЌдкбєМЋЗЂЩњЃЌЙЪbЮЊвѕРызгНЛЛЛФЄЃЌбєМЋЕФЕчМЋЗДгІЪНЮЊSO32--2e-+H2O=2H++ SO42-ЃЌЙЪД№АИЮЊЃКвѕЃЛSO32--2e-+H2O=2H++ SO42-ЁЃ

ЁОЬтФПЁПЯТСаЪЕбщВйзїЁЂЯжЯѓКЭНсТлОље§ШЗЃЌЧвДцдкЖдгІЙиЯЕЕФЪЧ
бЁЯю | ЪЕбщВйзї | ЪЕбщЯжЯѓ | НсТл |
A | НЋNaOHШмвКж№ЕЮЕЮМгЕНAlC13ШмвКжажСЙ§СП | ЯШВњЩњАзЩЋНКзДГСЕэЃЌКѓГСЕэШмНт | Al(OH)3ЪЧСНадЧтбѕЛЏЮя |
B | NaHCO3ШмвКгыNaAlOШмвКЛьКЯ | ЩњГЩАзЩЋГСЕэ | НсКЯH+ЕФФмСІЃКCO32->AlO2- |
C | ЯђЪЂгаNa2SiO3ЃЌШмвКЕФЪдЙмжаЕЮМг1ЕЮЗгЬЊЃЌШЛКѓж№ЕЮМгШыЯЁбЮЫсжСЙ§СП | ЪдЙмжаКьЩЋж№НЅЭЪШЅЃЌГіЯжАзЩЋФ§НК | ЗЧН№ЪєадЃКCl>Si |
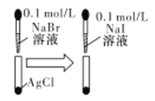
D |
| АзЩЋЙЬЬхЯШБфЮЊЕЛЦЩЋЃЌКѓБфЮЊЛЦЩЋ | ШмЖШЛ§ГЃЪ§ЃКKsp(AgCl)>Ksp(AgBr)>Ksp(AgI) |
A.AB.BC.CD.D