��Ŀ����
7����ѧ��Ӧ���������������仯����1����25�桢101kPa�£�1gҺ̬�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����22.68kJ�����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪCH3OH��l��+$\frac{3}{2}$O2��g���TCO2��g��+2H2O��l����H=-725.8 kJ•mol-1��
��2����֪�Ͽ�1mol H-H����1mol N-H����1mol N��N���ֱ���Ҫ��������436kJ��391kJ��946kJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪN2��g��+3H2��g��?2NH3��g����H=-92kJ•mol-1
��3����298K�£�C��Al�ĵ��ʸ�1mol��ȫȼ�գ��ֱ�ų�����a kJ��b kJ����֪һ�������£�Al�ܽ�C��CO2�û�������д�����û���Ӧ���Ȼ�ѧ����ʽ��4Al��s��+3CO2��g���T2Al2O3��s��+3C��s����H=-��4b-3a��kJ/mol��
��4��0.1mol����̬����ȼ�������飨B2H6����O2��ȼ�գ����ɹ�̬B2O3��Һ̬ˮ���ų�216.5kJ�����������Ȼ�ѧ����ʽΪB2H6��g��+3O2��g���TB2O3��s��+3H2O��l����H=-2165kJ/mol����֪��H2O��l��=H2O��g����H=+44kJ•mol-1����11.2L ����״���£�B2H6��ȫȼ��������̬ˮʱ�ų���������1016.5kJ��
���� ��1��ȼ������1mol��ȼ����ȫȼ�������ȶ�������ʱ�ų�����������25�桢101kPa�£�1g�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����22.68kJ��1mol�״���ȫȼ�����ɶ�����̼��Һ̬ˮ����725.8KJ��
��2����ѧ��Ӧ�У���ѧ�����������������γ��»�ѧ���ų����������ݷ���ʽ����ֱ����պͷų����������Դ˼��㷴Ӧ�Ȳ��ж����Ȼ��Ƿ��ȣ�
��3��������298K�£�C��Al�ĵ��ʸ�1mol��ȫȼ�գ��ֱ�ų�����aKJ��bKJ��˹���ɼ���õ������Ȼ�ѧ����ʽ��
��4��0.3mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5KJ����������1mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�2165KJ�����������ݸ�˹����д�����Ȼ�ѧ��Ӧ����ʽ����������ʵ����ʵ����뷴Ӧ�ų������������������
��� �⣺��1����25�桢101kPa�£�1g�״���CH3OH��ȼ������CO2��Һ̬ˮʱ����22.68kJ��1mol�״���ȫȼ�����ɶ�����̼��Һ̬ˮ����Ϊ22.68kJ��32=725.8KJ�����Լ״�ȼ���ȵ��Ȼ�ѧ����ʽΪCH3OH��l��+$\frac{3}{2}$O2��g���TCO2��g��+2H2O��l����H=-725.8 kJ•mol-1��
�ʴ�Ϊ��CH3OH��l��+$\frac{3}{2}$O2��g���TCO2��g��+2H2O��l����H=-725.8 kJ•mol-1��
��2���ڷ�ӦN2+3H2?2NH3�У�����3molH-H����1molN��N�������յ�����Ϊ3��436kJ+946kJ=2254kJ������2molNH3�����γ�6molN-H�����ų�������Ϊ6��391kJ=2346kJ�����յ������٣��ų��������࣬�÷�ӦΪ���ȷ�Ӧ���ų�������Ϊ2346kJ-2254kJ=92kJ��N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��?2NH3��g����H=-92kJ•mol-1��
�ʴ�Ϊ��N2��g��+3H2��g��?2NH3��g����H=-92kJ•mol-1��
��3���������д���Ȼ�ѧ����ʽ��
��C��s��+O2��g���TCO2��g����H=-a kJ•mol-1��
��4Al��s��+3O2��g���T2Al2O3��s����H=-4b kJ•mol-1��
���������û�̼�ķ�ӦΪ��4Al+3CO2$\frac{\underline{\;����\;}}{\;}$2Al2O3+3C��
���ݸ�˹���ɣ���-�١�3�ɵã�4Al��s��+3CO2 ��g���T2Al2 O3 ��s��+3C��s����H=-��4b-3a��kJ/mol��
�ʴ�Ϊ��4Al��s��+3CO2 ��g���T2Al2 O3 ��s��+3C��s����H=-��4b-3a��kJ/mol��
��4��0.1mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�216.5KJ����������1mol��̬����ȼ����������������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�2165KJ����������Ӧ���Ȼ�ѧ����ʽΪ��B2H6��g��+3O2��g���TB2O3��s��+3H2O��l����H=-2165kJ/mol��
��B2H6��g��+3O2��g���TB2O3��s��+3H2O��l����H=-2165kJ/mol��
��H2O��l����H2O��g������H=+44kJ/moL��
�ɸ�˹���ɿ�֪��+�ڡ�3�ã�B2H6��g��+3O2��g���TB2O3��s��+3H2O��g����H=-2033kJ/mol��
11.2L����״������0.5mol��������ȫȼ��������̬ˮʱ�ų���������2033kJ/mol��0.5mol=1016.5kJ��
�ʴ�Ϊ��B2H6��g��+3O2��g���TB2O3��s��+3H2O��l����H=-2165kJ/mol��1016.5��
���� ���⿼��ȼ���ȵ��Ȼ�ѧ����ʽ����д���ʱ����㣬��Ŀ�ѶȲ���ע���ʾ�Ȼ�ѧ����ʽ����дҪע�����ʾۼ�״̬�ͷ�Ӧ�ȵ����������⣮

| A�� | ��������ˮ���ܽ��Ա�KHCO3��K2CO3 | |
| B�� | ���ȶ���K2CO3��KHCO3 | |
| C�� | ���ᷴӦ�ų�CO2�Ŀ���KHCO3��K2CO3 | |
| D�� | ����������ֱ�������KHCO3��K2CO3��Ӧ������CO2һ���� |
| A�� | CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=55.65kJ/mol | |
| B�� | CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-55.65kJ/mol | |
| C�� | CH4��g��+2O2��g���TCO2��g��+2H2O��l����H=-890.4kJ/mol | |
| D�� | CH4��g��+2O2�TCO2+2H2O��H=890.4kJ/mol |
 �״�����Ҫ�Ļ���ԭ�ϣ��ֿɳ�Ϊȼ�ϣ����úϳ�������Ҫ�ɷ�ΪCO��CO2��H2���ڴ����������ºϳɼ״�������������Ӧ���£�
�״�����Ҫ�Ļ���ԭ�ϣ��ֿɳ�Ϊȼ�ϣ����úϳ�������Ҫ�ɷ�ΪCO��CO2��H2���ڴ����������ºϳɼ״�������������Ӧ���£���CO��g��+2H2��g��?CH3OH��g����H1
��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H2
��CO2��g��+H2��g��?CO��g��+H2O��g����H3
�ش��������⣺
��1����֪��Ӧ���е���صĻ�ѧ�������������£�������CO�е�̼����ΪC$\frac{\underline{\;��\;}}{\;}$O��
| ��ѧ�� | H-H | C-O | C$\frac{\underline{\;��\;}}{\;}$O | H-O | C-H |
| E/��kJ��mol-1�� | 436 | 343 | 1076 | 465 | 413 |
��2����25�桢101KPa�£�ÿ���ȼ��1g CH3OH���ָ���ԭ״̬�����ͷ�22.68KJ����������д����ʾ�״�ȼ���ȵ��Ȼ�ѧ��Ӧ����ʽ��CH3OH��l��+$\frac{3}{2}$O2��g��=CO2��g��+2H2O��l����H=-725.76kJ•mol-1��
��3������CO2��H2��Ӧ���ɺϳɶ����ѣ�CH3OCH3������KOHΪ�������Һ����ɶ����ѿ���ȼ�ϵ�أ��õ�ع���ʱ�为����Ӧʽ��CH3OCH3-12e-+16OH-=2CO32-+11H2O��
��4�������£��ö�����ȼ�ϵ�ص��600mL NaCl��Һ�������Ķ�����0.23g����������������1.344L����״��������Һ��pH=13��
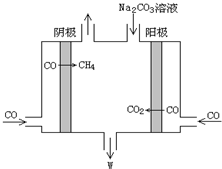
��5�����ö�����ȼ�ϵ�ص��CO�Ʊ�CH4��W������ԭ����ͼ��ʾ��������WNaHCO3�ǣ����������ӷ���ʽ��4CO+3CO32-+5H2O=6HCO3-+CH4����
| A�� | CO2��ˮ��Һ���Ե��磬CO2�ǵ���� | |
| B�� | ���ᱵ������ˮ����ȴ�ǵ���� | |
| C�� | �Ȼ�����Һ�ڵ����������µ���������Ӻ������� | |
| D�� | ����ˮ���ܵ���������ӵĻ����ﶼ���� |
| A�� | ���� | B�� | ˮ�� | C�� | ���� | D�� | �����ʵı��� |
 ��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l ����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ•mol-1��-283.0kJ•mol-1��-726.5kJ•mol-1����ش��������⣺
��ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪H2��g����CO��g����CH3OH��l ����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ•mol-1��-283.0kJ•mol-1��-726.5kJ•mol-1����ش��������⣺
 ��¯�����з����Ļ�����Ӧ֮һ��FeO��s��+CO��g��?Fe��s��+CO2��g��������Ӧ����
��¯�����з����Ļ�����Ӧ֮һ��FeO��s��+CO��g��?Fe��s��+CO2��g��������Ӧ����