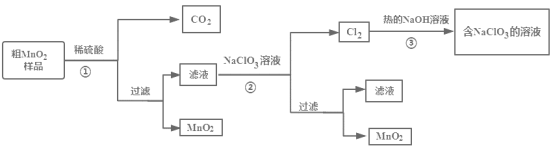
��Ŀ����
����Ŀ���л���ķ���ʽΪC4H9Br�������˵��������ܷ�������ת����ϵ��
B![]() A
A![]() �ף�C4H9Br��
�ף�C4H9Br��![]() D
D![]() E
E
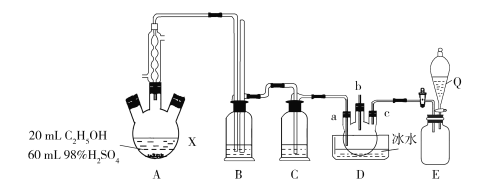
��֪��B�ܷ���������Ӧ���˴Ź���������ʾ�����������շ塣�Իش��������⣺
��1�������ƣ�____��������A�ķ�Ӧ������___��
��2��B������������ͭ����Һ��Ӧ�Ļ�ѧ����ʽ��____��
��3������NaOH����Һ���ȵĻ�ѧ����ʽΪ��___��
��4��A��ͬ��ͬ���칹���У����ܱ������������ʵĽṹ��ʽΪ___��
��5��D����E�Ļ�ѧ����ʽ��___��
���𰸡�2-��-1-����� ˮ�ⷴӦ��ȡ����Ӧ�� (CH3)2CHCHO+2Cu(OH)2+NaOH![]() (CH3)2CHCOONa+Cu2O��+3H2O (CH3)2CHCH2Br+NaOH
(CH3)2CHCOONa+Cu2O��+3H2O (CH3)2CHCH2Br+NaOH![]() (CH3)2C=CH2��+NaBr+H2O (CH3)3COH n CH2=C(CH3)2�� ��[-CH2-C(CH3)2-]��n
(CH3)2C=CH2��+NaBr+H2O (CH3)3COH n CH2=C(CH3)2�� ��[-CH2-C(CH3)2-]��n
��������
C4H9Br��NaOH��Һ���������·���ˮ������C4H9OH��C4H9OH��Cu�����������±���������C4H8O��C4H9Br��NaOH����Һ�����·�����ȥ��Ӧ����ϩ��C4H8��ϩ���ɷ����Ӿ۷�Ӧ���ݴ˻ش����⡣
��1��C4H9Br��NaOH��Һ���������·���ˮ������C4H9OH��C4H9OH��Cu�����������±���������C4H8O����C4H8O�ܷ���������Ӧ���˴Ź���������ʾ�����������շ壬˵����������ԭ�ӣ�����C4H8O�ĽṹʽΪ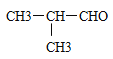 ����C4H9Br�ĽṹʽΪ��
����C4H9Br�ĽṹʽΪ��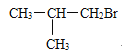 ������Ϊ2-��-1-����飻C4H9Br��NaOH����Һ�����·�����ȥ��Ӧ����ϩ��C4H8 ��
������Ϊ2-��-1-����飻C4H9Br��NaOH����Һ�����·�����ȥ��Ӧ����ϩ��C4H8 ��
��2��B������������ͭ����Һ��Ӧ����(CH3)2CHCOONa��������ͭ��ˮ���ʷ�Ӧ����ʽΪ��(CH3)2CHCHO+2Cu(OH)2+NaOH![]() (CH3)2CHCOONa+Cu2O��+3H2O��
(CH3)2CHCOONa+Cu2O��+3H2O��
��3��C4H9Br��NaOH����Һ�����·�����ȥ��Ӧ����ϩ��C4H8������ʽΪ��(CH3)2CHCH2Br+NaOH![]() (CH3)2C=CH2��+NaBr+H2O��
(CH3)2C=CH2��+NaBr+H2O��
��4��C4H9OH��ͬ���칹���У��ǻ�������̼ԭ����û����ԭ�ӣ����ܷ�������������Ϊ��(CH3)3COH��
��5��ϩ���ɷ����Ӿ۷�Ӧ������ʽΪ��n CH2=C(CH3)2�� ��[-CH2-C(CH3)2-]��n

 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�