��Ŀ����
����Ŀ���õ������(��Ҫ��![]() ��
��![]() )���������Ȼ������������������壬�乤���������£�
)���������Ȼ������������������壬�乤���������£�

��֪����![]() ��ˮ�����ɼ�ʽ�Ȼ�����
��ˮ�����ɼ�ʽ�Ȼ�����![]() ������ˮ��Һ�У�
������ˮ��Һ�У�![]() �ױ�����Ϊ
�ױ�����Ϊ![]() ��
��
��ش��������⣺
(1)�������ڱ��е�λ��Ϊ___________��
(2)��֪��ӦI�õ��ij�����![]() ����������ĽṹʽΪ_____����Ӧ�Ļ�ѧ����ʽΪ_______��
����������ĽṹʽΪ_____����Ӧ�Ļ�ѧ����ʽΪ_______��
(3)ͼ����Һ����Ҫ�ɷ�Ϊ_______________��_______________(д��ѧʽ)��
(4)����ʱһ�����Ũ��������ܽ⣬���û�ѧ����ʽ����Ҫ���ֽ���ԭ��______________��
(5)��������е������ռ��������Һ��Ӧ�����������ƣ�������һ�ּ������壬�÷�Ӧ�Ļ�ѧ����ʽΪ________��
���𰸡��������ڵ���A�� O��C��O Na2CO3+SnCl2 �� SnO��+2NaCl+CO2�� NaCl Na2CO3 SnCl2����ˮ�⣬����ƽ��SnCl2+H2O![]() Sn(OH)Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�� 2Sn+3NaOH+NaNO3 �� 2Na2SnO3+NH3��
Sn(OH)Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�� 2Sn+3NaOH+NaNO3 �� 2Na2SnO3+NH3��
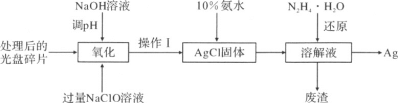
��������
��������е�Sn��SnO�����ᷴӦ����SnCl2��ϴ�Ӳ��ᾧ��õ�SnCl2���壬���þ����ܽ��������з�ֹSnCl2ˮ�⣬���������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ�������м���Sn�ۣ�Sn�ۿ��Ժ�H+������Ӧ��ʹ��Һ���Լ�������������ҺpH������Sn���Խ����������ɵ�Sn4+��ԭ��Sn2+������ֹSn2+������ΪSn4+�����˵�SnCl2��Һ�������м�̼���ƣ���SnԪ����SnO��ʽ������������Ӧ�ķ���ʽΪNa2CO3+SnCl2 �� SnO��+2NaCl+CO2��������ϴ�ӵô�����SnO����Ӧ����Һ������ΪNaCl��Na2CO3��SnO�м�ϡH2SO4����SnSO4��Һ����������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵ�ϵ�в������Ƶ�SnSO4���塣
(1)��Ԫ����̼Ԫ������ͬһ���壬������A�壬ԭ�Ӻ˵����Ϊ50����50281818��4����Sn���ڵ������ڣ��������ڱ��е�λ��Ϊ���������ڵ���A�壻
(2)��Ӧ���Ļ�ѧ����ʽΪNa2CO3+SnCl2 �� SnO��+2NaCl+CO2�������ɵ�����ΪCO2��CO2Ϊ���ۻ������ṹʽΪO��C��O��
(3)���Ϸ�����Һ������ΪNaCl��Na2CO3��
(4)����Ϣ��֪��SnCl2��ˮ�����ɼ�ʽ�Ȼ�����������ƽ��SnCl2+H2O![]() Sn(OH)Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⣻
Sn(OH)Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⣻
(5)��Ӧ��ΪSn��NaOH��NaNO3��������ΪNa2SnO3��NH3������Sn+NaOH+NaNO3��Na2SnO3+NH3�������ݵ�ʧ�����غ��Ԫ���غ㣬�ɵ�2Sn+3NaOH+NaNO3 �� 2Na2SnO3+NH3����