��Ŀ����
����Ŀ����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2������ȫ������ձ����ӡ�Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���Ϊ̽���÷�Ӧԭ������������ʵ�飺���ݻ�Ϊ1L���ܱ������У�����1mol CO2��3mol H2����500���·�����Ӧ��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)��ʵ����CO2��CH3OH(g)�����ʵ���(n)��ʱ��仯��ͼ��ʾ��
CH3OH(g)+H2O(g)��ʵ����CO2��CH3OH(g)�����ʵ���(n)��ʱ��仯��ͼ��ʾ��

(1)�ӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v(H2)=________________����ͼ�Ǹı��¶�ʱ��ѧ��Ӧ������ʱ��仯��ʾ��ͼ����÷�Ӧ������ӦΪ____________��Ӧ������ȡ������ȡ�����

(2)500��÷�Ӧ��ƽ�ⳣ��Ϊ______��������λС������������¶ȵ�800����У���ƽ��ʱ��Kֵ______�����������С�����䡱����
(3)500�������£����ijʱ�̣�CO2(g)��H2(g)��CH3OH(g)��H2O(g)��Ũ�Ⱦ�Ϊ0.5mol/L�����ʱv(��)______v(��)�����������������=������
(4)���д�ʩ��ʹ �������______��
�������______��
A�������¶� B����ԭ�����г���1molHe
C����ˮ��������ϵ�з���� D����С�����ݻ�������ѹǿ
���𰸡�0.225mol/(L��min) ���� 5.33 ��С �� CD
��������
��1����ͼ��֪��10min����ƽ�⣬ƽ��ʱ�״���Ũ�ȱ仯Ϊ0.75mol/L���ɷ���ʽ��֪������Ũ�ȱ仯���ڼ״���Ũ�ȱ仯��3��Ϊ2.25mol/L���ٽ��![]() ���㣻�����¶ȶ�ƽ���Ӱ��Ч������
���㣻�����¶ȶ�ƽ���Ӱ��Ч������
��2��ƽ�ⳣ�������������Ũ��ϵ������֮���뷴Ӧ���Ũ��ϵ������֮���ı�ֵ�������¶ȣ�ƽ�������ƶ���ƽ�ⳣ����С��
��3�����ݼ���Ũ���̺��¶��µ�ƽ�ⳣ���ȽϷ����жϷ�Ӧ���з���
��4��Ҫ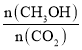 ����Ӧʹƽ��������Ӧ�����ƶ�������ƽ���ƶ�ԭ�����ѡ���жϡ�
����Ӧʹƽ��������Ӧ�����ƶ�������ƽ���ƶ�ԭ�����ѡ���жϡ�
��1����ͼ��֪��10min����ƽ�⣬ƽ��ʱ�״���Ũ�ȱ仯Ϊ0.75mol/L���ɷ���ʽCO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)��֪��������Ũ�ȱ仯���ڼ״���Ũ�ȱ仯����3����Ϊ0.75mol/L��3=2.25mol/L����v(H2)=
CH3OH(g)+H2O(g)��֪��������Ũ�ȱ仯���ڼ״���Ũ�ȱ仯����3����Ϊ0.75mol/L��3=2.25mol/L����v(H2)=![]() =0.225mol/(L��mon)������ͼʾ��Ϣ��֪�������¶ȣ�ƽ�����淴Ӧ�����ƶ�����÷�Ӧ������ӦΪ���ȷ�Ӧ���ʴ�Ϊ��0.225mol/(L��min)�����ȣ�
=0.225mol/(L��mon)������ͼʾ��Ϣ��֪�������¶ȣ�ƽ�����淴Ӧ�����ƶ�����÷�Ӧ������ӦΪ���ȷ�Ӧ���ʴ�Ϊ��0.225mol/(L��min)�����ȣ�
��2���ɣ�1����֪ƽ��ʱ����ֵ�Ũ��c(CO2)=0.25mol/L��c(CH3OH)=c(H2O)=0.75mol/L����c(H2)=3mol/L-0.75mol/L��3=0.75mol/L������ƽ�ⳣ��K=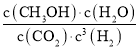 =
=![]() =5.3�������¶ȣ�ƽ�����淴Ӧ�����ƶ���ƽ�ⳣ�����С���ʴ�Ϊ��5.3����С��
=5.3�������¶ȣ�ƽ�����淴Ӧ�����ƶ���ƽ�ⳣ�����С���ʴ�Ϊ��5.3����С��
��3�� CO2(g)��H2(g)��CH3OH(g)��H2O(g)��Ũ�Ⱦ�Ϊ0.5mol/L����Ũ����Q=![]() =4��K=5.33��˵����Ӧ�������v(��)��v(��)���ʴ�Ϊ������
=4��K=5.33��˵����Ӧ�������v(��)��v(��)���ʴ�Ϊ������
��4��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)Ϊ���������С�ķ��ȷ�Ӧ����
CH3OH(g)+H2O(g)Ϊ���������С�ķ��ȷ�Ӧ����
A���÷�ӦΪ���ȷ�Ӧ���������¶ȣ�ƽ�����淴Ӧ�����ƶ���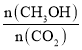 ��С��A�����
����A�����
B. ��ԭ�����г���1 mol He����Ӧ���Ũ�ȱ��ֲ��䣬ƽ�ⲻ�ƶ���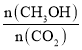 ��ֵ���ֲ��䣬B�����
��ֵ���ֲ��䣬B�����
C. ��ˮ��������ϵ�з������ƽ��������Ӧ�����ƶ���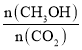 ��ֵ����C����ȷ��
��ֵ����C����ȷ��
D. ��С�����ݻ�������ѹǿ��ƽ��������Ӧ�����ƶ���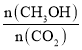 ��ֵ����D����ȷ��
��ֵ����D����ȷ��
�ʴ�ΪCD��

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�