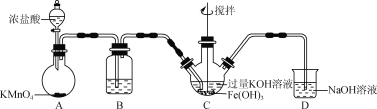
��Ŀ����
����Ŀ��(1)��֪�ɽ������Ƶ������ƣ����ö��ַ�����a.4Na��O2===2Na2O��b.4Na��CO2===2Na2O��C��c.2NaNO2��6Na===4Na2O��N2����
�����������ַ����У���õķ�����________(�����)��ԭ����________________��
��������Ӧc��NaNO2��________��������1 mol NaNO2��Ӧʱ������ת�Ƶ���Ŀ��________________________________________________________________________��
(2)���ý����ƺͿ����Ʊ����Ƚϸߵ�Na2O2�������õ�װ�����¡��ش���������(ע��Na2O2������H2O��CO2��Ӧ)��
��װ�â���ʢ�ŵ�ҩƷ��________����������____________________________��

�����涨�����������������ң������ʵ��װ��ʱ�������ӿڵı����ĸ(a��b����)˳��������________��________��________��________��________��________��________��
��װ�â������____________________________________________________��
�ܲ�����ͨ�����ͼ��ȵ�˳��Ϊ________________________________________��
���𰸡�c ��������ֻ��Na2O�ǹ��壬����һ�ֲ���N2������Χ�����е�O2�ž�����ֹNa2O������������Na2O2 ���� 1.806��1024 ����������Һ ���յ���Ŀ����еĶ�����̼ g��h��e��f��a(��b)��b(��a)��c ��ֹ�����е�ˮ�ֺͶ�����̼����װ�â� ��ͨһ��ʱ��Ŀ����ټ���װ�â�
��������
��1������Na2O���ȶ��ױ�������������������𣻸��ݵ�Ԫ�صĻ��ϼ۱仯�����жϺͼ��㣻
��2���ý����ƺͿ����Ʊ����Ƚϸߵ�Na2O2����Ҫ�����е��������Ƽ��ȷ�Ӧ���ɹ������ƣ������е�ˮ�����Ͷ�����̼��Ҫ��ȥ���ѿ���ͨ�������տ����еĶ�����̼���壬��ͨ��װ�â�����ˮ������ͨ��װ�â�����ƺ�������Ӧ�����Ӣ��ֹ�����еĶ�����̼��ˮ��������װ�âò��������Ĺ������ƣ��ݴ˽��
��1����Na2O���ȶ��ױ��������������������������ַ�����õ���c����Ϊ��������ֻ��Na2O�ǹ��壬����һ�ֲ���N2������Χ�����е�O2�ž�����ֹNa2O������������Na2O2��
�ڷ�Ӧ2NaNO2��6Na��4Na2O��N2���е�Ԫ�ػ��ϼ۴�+3�۽��͵�0�ۣ��õ�3�����ӣ���������������������������1 mol NaNO2��Ӧʱ������ת�Ƶ���Ŀ��1mol��3��6.02��1023/mol��1.806��1024��
��2�������ڿ����еĶ�����̼��ˮ����Ҳ��������Ʒ�Ӧ�����Ź������Ƶ��Ʊ�������Ҫ��ȥ�����װ�â���ʢ��NaOH��Һ�������������յ���Ŀ����еĶ�����̼��
�ڰѿ���ͨ�������տ����еĶ�����̼���壬��ͨ��װ�â�����ˮ������ͨ��װ�â�����ƺ�������Ӧ�����Ӣ��ֹ�����еĶ�����̼��ˮ��������װ�â�������ʵ��װ��ʱ����������ȷ����˳��Ϊ��������������ӿڵı����ĸ˳��Ϊ��������g��h��e��f��a����b����b����a����c��
��װ�â���ʢ�ż�ʯ�������ն�����̼��ˮ�������������Ƿ�ֹ�����еĶ�����̼��ˮ��������װ�â�
��ʵ��ʱ��ͨ�������������ٽ��м��ȣ��������ɵĹ������ƺͶ�����̼��ˮ������Ӧ����̼���Ƶ����ʡ�

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�����Ŀ�����ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���á�
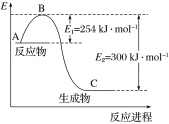
(1)��ͼ��N2(g)��H2(g)��Ӧ����1 mol NH3(g)�����������ı仯ʾ��ͼ����д��N2��H2��Ӧ���Ȼ�ѧ����ʽ��______________��
(2)����֪�������ݣ�
��ѧ�� | H��H | N��N |
����/kJ��mol��1 | 435 | 943 |
�Ը��ݱ��м�ͼ�����ݼ���N��H�ļ��ܣ�________ kJ��mol��1��
(3)��NH3����ԭNOx���������������������Ⱦ����֪��
4NH3(g)��3O2(g)=2N2(g)��6H2O(g) ��H1����a kJ��mol��1��
N2(g)��O2(g)===2NO(g)����H2����b kJ��mol��1��
��1 mol NH3��ԭNO��N2����÷�Ӧ�����еķ�Ӧ����H3��________ kJ��mol��1(�ú�a��b��ʽ�ӱ�ʾ)��
(4)��̼����(��Ҫָ����CO2)�ڽ������������ŷ��о�����Ҫ�����á�ĿǰNH3��(NH4)2CO3�Ѿ���������ҵ��̼����������CO2�ɷ������¿��淴Ӧ��
��Ӧ��2NH3(l)��H2O(l)��CO2(g) ![]() (NH4)2CO3(aq) ��H1
(NH4)2CO3(aq) ��H1
��Ӧ��NH3(l)��H2O(l)��CO2(g) ![]() NH4HCO3(aq)����H2
NH4HCO3(aq)����H2
��Ӧ��(NH4)2CO3(aq)��H2O(l)��CO2(g) ![]() 2NH4HCO3(aq)����H3
2NH4HCO3(aq)����H3
����H3����H1����H2֮��Ĺ�ϵ����H3��________��