��Ŀ����
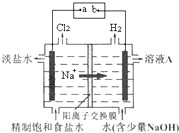
��ҵ���õ�ⱥ��ʳ��ˮ�ķ��������������ռ
��1��ʳ������ˮ�������룬����뷽��ʽΪ______
��2�����ʳ��ˮ��ԭ���ǣ�
______NaCl+______H2O
______H2��+______Cl2��+______NaOH
����������ѧ����ʽ�и����ʵĻ�ѧ���������ں����ϣ�
��3��2004��4��15�գ�ij��������������й©�¼���������Ա����NaOH��Һ�γ�ҺĻ����Χ������й©���������䷴Ӧԭ��Ϊ______�������ӷ���ʽ��ʾ����
��4���¼���������������߷�ӳ��ʱ����������·���ɫ�ˣ���ԭ����______����ϻ�ѧ����ʽ���ͣ���
��1��ʳ������ˮ�������룬����뷽��ʽΪ______
��2�����ʳ��ˮ��ԭ���ǣ�
______NaCl+______H2O
| ||
����������ѧ����ʽ�и����ʵĻ�ѧ���������ں����ϣ�
��3��2004��4��15�գ�ij��������������й©�¼���������Ա����NaOH��Һ�γ�ҺĻ����Χ������й©���������䷴Ӧԭ��Ϊ______�������ӷ���ʽ��ʾ����
��4���¼���������������߷�ӳ��ʱ����������·���ɫ�ˣ���ԭ����______����ϻ�ѧ����ʽ���ͣ���
��1���Ȼ�����ǿ����ʣ���ˮ������������ȫ������������ӣ���������뷽��ʽΪ��NaCl=Na++Cl-��
�ʴ�Ϊ��NaCl=Na++Cl-��
��2����ⱥ��ʳ��ˮ�����ռNaOH����ͬʱ����������Cl2������������������������������ʼ��ƽ������1����������Ҫ�õ�2�����ӣ�����1����������Ҫʧȥ2�����ӣ���ʧ������ȣ�Ȼ��۲취��ƽ���÷�Ӧ�Ļ�ѧ����ʽΪ��2NaCl+2H2O
2NaOH+Cl2��+H2����
�ʴ�Ϊ��2NaCl+2H2O
2NaOH+Cl2��+H2����
��3������������������Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ�����ӷ�Ӧ����ʽΪ��Cl2+2OH-=Cl-+ClO-+H2O��
�ʴ�Ϊ��Cl2+2OH-=Cl-+ClO-+H2O��
��4��������ˮ��Ӧ���ɴ����ᣬ��Ӧ����ʽΪ��Cl2+H2O=HCl+HClO��HClO����ǿ�����ԣ���Ư�ף�������ʹʪ�·���ɫ��
�ʴ�Ϊ��Cl2+H2O=HCl+HClO��HClO����ǿ�����ԣ���Ư����ɫ���ʣ�
�ʴ�Ϊ��NaCl=Na++Cl-��
��2����ⱥ��ʳ��ˮ�����ռNaOH����ͬʱ����������Cl2������������������������������ʼ��ƽ������1����������Ҫ�õ�2�����ӣ�����1����������Ҫʧȥ2�����ӣ���ʧ������ȣ�Ȼ��۲취��ƽ���÷�Ӧ�Ļ�ѧ����ʽΪ��2NaCl+2H2O
| ||
�ʴ�Ϊ��2NaCl+2H2O
| ||
��3������������������Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ�����ӷ�Ӧ����ʽΪ��Cl2+2OH-=Cl-+ClO-+H2O��
�ʴ�Ϊ��Cl2+2OH-=Cl-+ClO-+H2O��
��4��������ˮ��Ӧ���ɴ����ᣬ��Ӧ����ʽΪ��Cl2+H2O=HCl+HClO��HClO����ǿ�����ԣ���Ư�ף�������ʹʪ�·���ɫ��
�ʴ�Ϊ��Cl2+H2O=HCl+HClO��HClO����ǿ�����ԣ���Ư����ɫ���ʣ�

��ϰ��ϵ�д�
�����Ŀ