��Ŀ����
����Ŀ����(Mo)��һ����Ҫ�Ĺ��ɽ���Ԫ��,�������ϼ�Ϊ+6��+5��+4,������㷺����ұ�𡢻�е���졢���ӡ�������һЩ�߿Ƽ�����������(Na2MoO4)��һ����������ȴˮϵͳ�Ľ�����ʴ��,Ҳ����������������ī�����ʡ�������ϵȡ���ͼ�ǻ����������Ʊ�������������Ƶ���Ҫ����ͼ,��֪�⾫�����Ҫ�ɷ�ΪMoS2,����������ˮ��
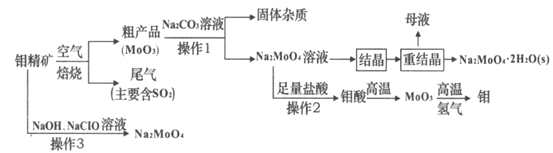
(1)MoS2����ʱ��Ӧ�Ļ�ѧ����ʽΪ________��������β���Ի�������ҪΣ����_______��
(2)����1��,�ֲ�Ʒ�е�MoO3��Na2CO3��Һ��ַ�Ӧ��,����Na2MoO4������һ������,�����ʵĵ���ʽΪ_____���ֲ�Ʒ������ּ�������õļ��Һ�в������ӵ�Ũ��:c(MoO42-)=0.40mol��L-1��c(SO42-)=0.05mol��L-1���ᾧǰӦ�ȳ�ȥSO42-,�����Ǽ���Ba(OH)2���塣�������Ba(OH)2�������Һ�������,��BaMoO4,��ʼ����ʱ,SO42-��ȥ����Ϊ___����֪:Ksp(BaSO4)=1.1��10-10,Ksp(BaMoO4)=4.0��10-8
(3)�������в���2Ϊ����,�����ʵ����ģ��ò���ʱ,������Һ������������,��ʵ������ĽǶȷ���,���ܵ�ԭ����_______________________________��
(4)�ؽᾧ�õ���ĸҺ�������´��ؽᾧʱ�ظ�ʹ��,���ﵽһ����������뾻������,ԭ��______________________________________��
(5)��ҵ����MoO3�Ʊ�Mo��Ҳ�������ȷ�Ӧ,д���÷�Ӧ�ķ���ʽ_______________��
(6)����3�ڼ���������,���⾫����뵽������NaC1O��Һ��,Ҳ�����Ʊ������ơ��÷�Ӧ�����ӷ���ʽΪ___________________________________��
���𰸡� ![]() �γ�����
�γ����� ![]() 97.8% ����ʱ©���е�Һ�������ֽ��Ե���������ڵ�����ֽһ��Ū����ֽ ʹ��һ��������ĸҺ�����ʵ�Ũ�������ؽᾧ���������ʣ�Ӱ���Ʒ����
97.8% ����ʱ©���е�Һ�������ֽ��Ե���������ڵ�����ֽһ��Ū����ֽ ʹ��һ��������ĸҺ�����ʵ�Ũ�������ؽᾧ���������ʣ�Ӱ���Ʒ���� ![]()
![]()
����������������ͼ��֪��MoS2����ʱ����MoO3��SO2����Ӧ�Ļ�ѧ����ʽΪ![]() ����Ӧ������SO2�ŷŵ������У��γ����ꣻ��ȷ�𰸣�
����Ӧ������SO2�ŷŵ������У��γ����ꣻ��ȷ�𰸣�![]() ���γ����ꡣ
���γ����ꡣ
(2)����MoO3��Na2CO3��Һ��ַ�Ӧ����Na2MoO4��֪�÷�ӦΪ��������ԭ��Ӧ������ԭ���غ��֪����Ӧ��������һ������Ϊ������̼������ʽΪ![]() ������Ksp(BaMoO4)=c(Ba2+)��c(MoO42-)=4.0��10-8����c(MoO42-)=0.40mol��L-1ʱ��c(Ba2+)=10-7 mol��L-1������Ksp(BaSO4)= c(Ba2+)��c��SO42-��=1.1��10-10,��c(Ba2+)=10-7 mol��L-1������ʽ��c��SO42-��=2.2��10-2 mol��L-1���������ᱵ��������������ӵ�Ũ��Ϊ0.05-2.2��10-2 mol��L-1����BaMoO4��ʼ����ʱSO42-��ȥ����Ϊ��0.05-2.2��10-2��/0.05��100%=97.8% ����ȷ����
������Ksp(BaMoO4)=c(Ba2+)��c(MoO42-)=4.0��10-8����c(MoO42-)=0.40mol��L-1ʱ��c(Ba2+)=10-7 mol��L-1������Ksp(BaSO4)= c(Ba2+)��c��SO42-��=1.1��10-10,��c(Ba2+)=10-7 mol��L-1������ʽ��c��SO42-��=2.2��10-2 mol��L-1���������ᱵ��������������ӵ�Ũ��Ϊ0.05-2.2��10-2 mol��L-1����BaMoO4��ʼ����ʱSO42-��ȥ����Ϊ��0.05-2.2��10-2��/0.05��100%=97.8% ����ȷ����![]() �� 97.8%��
�� 97.8%��
(3)�������в���2Ϊ���ˣ�������Һ�����������ǣ����ܵ�ԭ���ǹ���ʱ©���е�Һ�������ֽ��Ե���������ڵ�����ֽһ��Ū����ֽ����ȷ��������ʱ©���е�Һ�������ֽ��Ե���������ڵ�����ֽһ��Ū����ֽ��
(4)�ؽᾧ�õ���ĸҺ�������´��ؽᾧʱ�ظ�ʹ�ã����ﵽһ����������뾻��������ԭ��ʹ��һ��������ĸҺ�����ʵ�Ũ�������ؽᾧ���������ʣ�Ӱ���Ʒ��������ȷ����ʹ��һ��������ĸҺ�����ʵ�Ũ�������ؽᾧ���������ʣ�Ӱ���Ʒ���ȡ�
(5)��������MoO3�����·�Ӧ������������Mo����Ӧ�ķ���ʽ��![]() ����ȷ�𰸣�
����ȷ�𰸣�![]() ��
��
(6)�������ƾ���ǿ�����ԣ��ܹ���MoS2�ڼ��Ի���������ΪMnO4- ��SO42-����Ӧ�����ӷ���ʽΪ![]() ����ȷ�𰸣�
����ȷ�𰸣�![]() ��
��