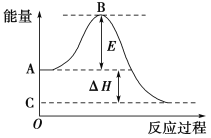
��Ŀ����
����Ŀ����ⷨ��ȡ�й㷺��;��Na2FeO4,ͬʱ��ȡ����:Fe+2H2O+2OH-![]() FeO42-+3H2��,����ԭ����ͼ(a)��ʾ,�������ҺΪ����������Һ,������������ҺŨ�ȹ���,���缫����������ɫɫ���ʡ�C(Na2FeO4)���ʼC(NaOH)�ı仯��ͼ(b)��ʾ����֪Na2FeO4ֻ��ǿ�����������ȶ�,�ױ�H2��ԭ������˵������ȷ����
FeO42-+3H2��,����ԭ����ͼ(a)��ʾ,�������ҺΪ����������Һ,������������ҺŨ�ȹ���,���缫����������ɫɫ���ʡ�C(Na2FeO4)���ʼC(NaOH)�ı仯��ͼ(b)��ʾ����֪Na2FeO4ֻ��ǿ�����������ȶ�,�ױ�H2��ԭ������˵������ȷ����
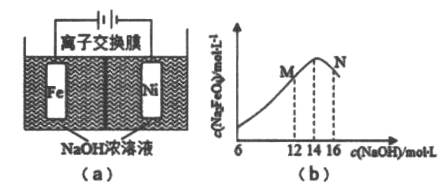
A. ���һ��ʱ���,������c(OH-)����
B. ��·�о���0.2mol����ʱ,����������H2 2.24L(��״��)
C. M��C(Na2FeO4)�������ֵ��ԭ�������缫����Fe(OH)3����
D. �����ĵ缫��Ӧ:Feһ6e-+8OH-===FeO42-+4H2O
���𰸡�C
���������������⣬�����Դ�����������������ص�������������ӦʽΪ��Fe-6e-+8OH-=FeO![]() +4H2O���������У�����������OH-��c(OH)���ͣ�A��ȷ�����缫�����ݲ�����������ӦʽΪ��2H2O+2e-=H2��+2OH-����·�о���0.2mol����ʱ,����������H2 2.24L(��״��)��B��ȷ����������Na2FeO4ֻ��ǿ�����������ȶ�����M�㣬c(OH-)�ͣ�Na2FeO4�ȶ��Բ�ҷ�Ӧ��������M��C(Na2FeO4)�������ֵ��C���������缫����������Ӧ��������ӦʽΪ��Fe-6e-+8OH-=FeO
+4H2O���������У�����������OH-��c(OH)���ͣ�A��ȷ�����缫�����ݲ�����������ӦʽΪ��2H2O+2e-=H2��+2OH-����·�о���0.2mol����ʱ,����������H2 2.24L(��״��)��B��ȷ����������Na2FeO4ֻ��ǿ�����������ȶ�����M�㣬c(OH-)�ͣ�Na2FeO4�ȶ��Բ�ҷ�Ӧ��������M��C(Na2FeO4)�������ֵ��C���������缫����������Ӧ��������ӦʽΪ��Fe-6e-+8OH-=FeO![]() +4H2O��D��ȷ����ȷѡ��C��
+4H2O��D��ȷ����ȷѡ��C��