��Ŀ����
(12��)�ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����塣��ԭ��Ϊ��
N2(g)+3H2(g) 2NH3(g) ��H=" -92.4KJ/mol " ���ݴ˻ش��������⣺
2NH3(g) ��H=" -92.4KJ/mol " ���ݴ˻ش��������⣺
��1�������йظ÷�Ӧ���ʵ���������ȷ���ǣ�ѡ����ţ� ��
a�������¶ȿ����������Ӱٷ������ӿ췴Ӧ����
b������ѹǿ�����������Ӱٷ��������Բ����Լӿ췴Ӧ����
c��ʹ�ô�������ʹ��Ӧ�����ƽ���������ߣ��ӿ췴Ӧ����
d��������һ��������£����������ı������С���Է�Ӧ����������Ӱ��
��2���ٸ÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK=_______________________��
�ڸ����¶ȶԻ�ѧƽ���Ӱ����ɿ�֪�����ڸ÷�Ӧ���¶�Խ�ߣ���ƽ�ⳣ����ֵԽ_____ ��
��3��ij�¶��£�����10 mol N2��30 mol H2�������Ϊ10 L���ܱ������ڣ���Ӧ�ﵽƽ��״̬ʱ�����ƽ���������а����������Ϊ20%������¶��·�Ӧ��K=___________�����÷�����ʾ������˵���÷�Ӧ�ﵽ��ѧƽ��״̬���� ������ĸ����
a�������ڵ��ܶȱ��ֲ��� b��������ѹǿ���ֲ���
c��������N2����2������NH3�� d�����������c��NH3������
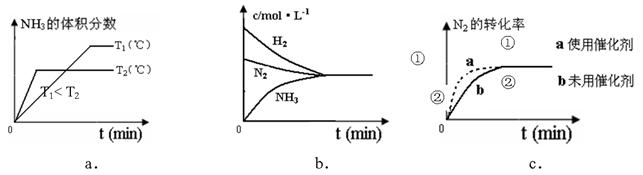
��4�����ںϳɰ���Ӧ���ԣ������й�ͼ��һ����ȷ���ǣ�ѡ����ţ�_____________��
��5����ͬ�¶��£��к����ܱ�����A�ͺ�ѹ�ܱ�����B���������о�����1mol N2��3 molH2����ʱ�������������ȡ���һ�������·�Ӧ�ﵽƽ��״̬��A��NH3���������Ϊa���ų�����Q1 kJ��
B��NH3���������Ϊb���ų�����Q2 kJ����a_____b(��>��=��<)�� Q1_____ Q2(��>��=��<)�� Q1_____92.4(��>��=��<)��
N2(g)+3H2(g)
 2NH3(g) ��H=" -92.4KJ/mol " ���ݴ˻ش��������⣺
2NH3(g) ��H=" -92.4KJ/mol " ���ݴ˻ش��������⣺��1�������йظ÷�Ӧ���ʵ���������ȷ���ǣ�ѡ����ţ� ��
a�������¶ȿ����������Ӱٷ������ӿ췴Ӧ����
b������ѹǿ�����������Ӱٷ��������Բ����Լӿ췴Ӧ����
c��ʹ�ô�������ʹ��Ӧ�����ƽ���������ߣ��ӿ췴Ӧ����
d��������һ��������£����������ı������С���Է�Ӧ����������Ӱ��
��2���ٸ÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK=_______________________��
�ڸ����¶ȶԻ�ѧƽ���Ӱ����ɿ�֪�����ڸ÷�Ӧ���¶�Խ�ߣ���ƽ�ⳣ����ֵԽ_____ ��
��3��ij�¶��£�����10 mol N2��30 mol H2�������Ϊ10 L���ܱ������ڣ���Ӧ�ﵽƽ��״̬ʱ�����ƽ���������а����������Ϊ20%������¶��·�Ӧ��K=___________�����÷�����ʾ������˵���÷�Ӧ�ﵽ��ѧƽ��״̬���� ������ĸ����
a�������ڵ��ܶȱ��ֲ��� b��������ѹǿ���ֲ���
c��������N2����2������NH3�� d�����������c��NH3������
��4�����ںϳɰ���Ӧ���ԣ������й�ͼ��һ����ȷ���ǣ�ѡ����ţ�_____________��

��5����ͬ�¶��£��к����ܱ�����A�ͺ�ѹ�ܱ�����B���������о�����1mol N2��3 molH2����ʱ�������������ȡ���һ�������·�Ӧ�ﵽƽ��״̬��A��NH3���������Ϊa���ų�����Q1 kJ��
B��NH3���������Ϊb���ų�����Q2 kJ����a_____b(��>��=��<)�� Q1_____ Q2(��>��=��<)�� Q1_____92.4(��>��=��<)��
(12�֣�ÿ��2�֣��ڣ�5����ÿ��1��)
��1��ad ��2�֣�
��2����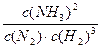 ��1�֣�
��1�֣�
��С (1��)
��3��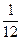 ��1�֣� ��b��d ��ȫ��2�֣�ѡ��1����1�֣���ѡ0�֣�
��1�֣� ��b��d ��ȫ��2�֣�ѡ��1����1�֣���ѡ0�֣�
��4��ac ������������5�� a<b Q1<Q2 Q1< 92.4
��1��ad ��2�֣�
��2����
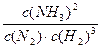 ��1�֣�
��1�֣���С (1��)
��3��
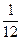 ��1�֣� ��b��d ��ȫ��2�֣�ѡ��1����1�֣���ѡ0�֣�
��1�֣� ��b��d ��ȫ��2�֣�ѡ��1����1�֣���ѡ0�֣���4��ac ������������5�� a<b Q1<Q2 Q1< 92.4
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 �鷽���� ��
�鷽���� ��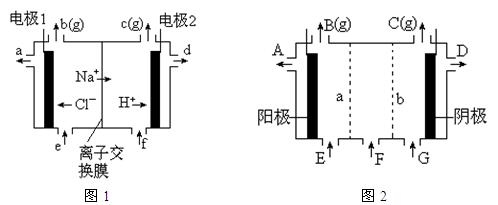
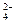 �����ϸߣ��������ӱ�ʽ����ȥSO
�����ϸߣ��������ӱ�ʽ����ȥSO 2NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa��
2NH3(g) ��H����92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶá��ҹ��ϳɰ���ҵĿǰ����������Ϊ������������ý���¶ȣ�400��500�棬ѹǿ��30��50MPa�� ( )
( )