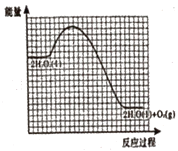
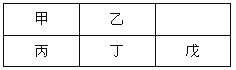
��Ŀ����
����Ŀ����֪ij��84����Һ��ƿ�岿�ֱ�ǩ��ͼ��ʾ���á�84����Һ��ͨ��ϡ��100�������֮�ȣ���ʹ�á���ش��������⣺

��1���á�84����Һ�������ʵ���Ũ��ԼΪ________mol��L��1��������С�����һλ��
��2��ijͬѧȡ100 mL�á�84����Һ����ϡ�ͺ�����������ϡ�ͺ����Һ��c��Na������________mol��L��1��
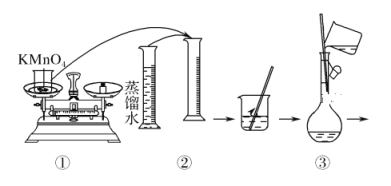
��3����ͬѧ���ĸá�84����Һ�����䷽������NaClO��������480 mL��NaClO��������Ϊ25%������Һ������˵����ȷ����________������ĸ����
A������ƿ������ˮϴ����Ӧ��ɺ����������Һ����
B�����ƹ����У�δ������ˮϴ���ձ��Ͳ��������ܵ��½��ƫ��
C����Ҫ����NaClO���������Ϊ143.0 g
��4����84����Һ����ϡ������ʹ�ÿ���ǿ����������ij����С����Ա��98%���ܶ�Ϊ1.84 g��cm��3����Ũ��������2 L 2.3 mol��L��1��ϡ����������ǿ��84����Һ��������������
�������Ƶ�ϡ�����У�H�������ʵ���Ũ��Ϊ________mol��L��1��
������Ũ��������Ϊ________mL��
���𰸡�4.0 0.04 B 4.6 250
��������
��1������![]() ���㣻
���㣻
��2������ϡ���������ʵ����ʵ���������
��3������480 mL��NaClO��������Ϊ25%������Һ��Ҫ500mL����ƿ�����һ�����ʵ���Ũ����Һ���Ƶ�ԭ���������
��4�����������Ƕ�Ԫǿ����������ӵ�Ũ�ȣ�����ϡ��������������ʵ������������ҪŨ����������
��1����![]() �ã�c(NaClO)��1000��1.19��25%/74.5 g��mol��1��4.0 mol��L��1��
�ã�c(NaClO)��1000��1.19��25%/74.5 g��mol��1��4.0 mol��L��1��
��2��ϡ��ǰ����Һ��NaClO�����ʵ������䣬��ϡ��100����c(NaClO)��0.04 mol��L��1��c(Na��)��c(NaClO)��0.04 mol��L��1��
��3��A�����ƹ�������Ҫ����ˮ�����Ծ�ϴ�Ӹɾ�������ƿ���غ�ɺ���ʹ�ã�������ƿ���ܺ�ɣ�A����
B��δϴ���ձ��Ͳ��������������Ƶ���Һ�����ʵ����ʵ�����С�����ƫ�ͣ�B��ȷ��
C��Ӧѡȡ500 mL������ƿ�������ƣ�Ȼ��ȡ��480 mL���ɣ�������ҪNaClO������Ϊ0.5 L��4.0 mol��L��1��74.5 g��mol��1��149.0 g��C����
��ѡB��
��4���ٸ���H2SO4����ɿ�֪����Һ��c(H��)��2c(H2SO4)��4.6 mol��L��1��
��2 L 2.3 mol��L��1��ϡ���������ʵ����ʵ���Ϊ2 L��2.3 mol��L��1��4.6 mol������Ҫ98%(�ܶ�Ϊ1.84 g��cm��3)��Ũ��������ΪV mL�����У�VmL��1.84g/mL��98%/98g��mol��1��4.6 mol�����V��250��

 ��У����ϵ�д�
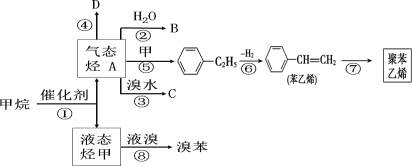
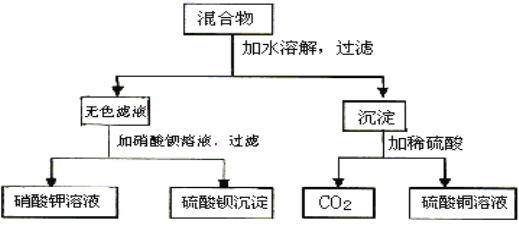
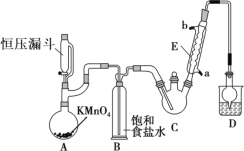
��У����ϵ�д�����Ŀ��������ȩ(CCl3CHO)������ũҩ��ҽҩ����Ҫ�м��壬ʵ�����Ʊ�������ȩ�ķ�Ӧװ��ʾ��ͼ(����װ��δ����)���й��������£�
���Ʊ���Ӧԭ����C2H5OH��4Cl2��CCl3CHO��5HCl
��������ʵ���Է��������������������ʣ�
��Է������� | �۵�/�� | �е�/�� | �ܽ��� | |
C2H5OH | 46 | ��114.1 | 78.3 | ��ˮ���� |
CCl3CHO | 147.5 | ��57.5 | 97.8 | ������ˮ���Ҵ� |
CCl3COOH | 163.5 | 58 | 198 | ������ˮ���Ҵ���������ȩ |
C2H5Cl | 64.5 | ��138.7 | 12.3 | ����ˮ���������Ҵ� |
(1)��ѹ©����ʢ�ŵ��Լ���������_____��ʢ��KMnO4������������_____��
(2)��Ӧ������C2H5OH��HCl���ܻ����ɸ�����C2H5Cl��ͬʱCCl3CHO(������ȩ)Ҳ�ܱ������������������CCl3COOH(��������)��д��������ȩ������������������������Ļ�ѧ����ʽ��_____��
(3)����������д���һ��ȱ����_____����������ĺ����_____��װ��B��������______��
(4)��Ӧ��������������Ƚ�C�еĻ������ȴ�����£����÷�Һ�ķ���������������ᡣ����Ϊ�˷����Ƿ����_____(���ǻ��)��ԭ����_____��
(5)�ⶨ��Ʒ���ȣ���ȡ��Ʒ0.36g��ɴ�����Һ������0.1000molL1�����Һ20.00mL���ټ�������Na2CO3��Һ����Ӧ��ȫ�����������Һ��pH��������0.02000molL1Na2S2O3��Һ�ζ����յ㡣��������ƽ��ʵ�飬���ƽ������Na2S2O3��Һ20.00mL�����Ʒ�Ĵ���Ϊ_____(������������λ��Ч����)���ζ�ԭ����CCl3CHO+OH-=CHCl3+HCOO-��HCOO-+I2=H++2I-+CO2��I2+2S2O32-=2I-+S4O62-
����Ŀ��̽���糡�������������ӵ�Ǩ�ơ�a��b��c��d ��Ϊʯī�缫���缫���4cm����pH��ֽ�ò�ͬŨ��Na2SO4��Һ�����ʪ����������ʵ�飺
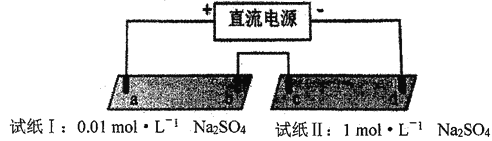
ʵ������
ʱ�� | ��ֽI | ��ֽII |
lmin | a��������ֽ��죬b��������ֽ���� | c��������ֽ��죬d���������� |
10min | ��ɫ������ɫ���������м���չ������ʱ��ɫ��Լ2.7cm����ɫ��Լ1.3cm | ������ɫ��Χ�������ԣ���ֽ����Ϊ��ɫ |
����˵������ȷ����
A. d��������ֽ����
B. a��������ֽ����ԭ���ǣ�2H2O+2e-= H2��+2OH-
C. �Ա���ֽI����ֽII������˵�������Ũ�Ȼ���Ӱ��H+��OH-��Ǩ��
D. ��ֽI������˵�����˻�����H+��Ǩ�����ʱ�OH-��