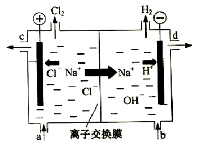
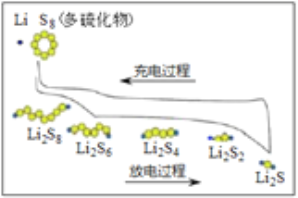
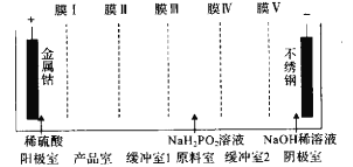
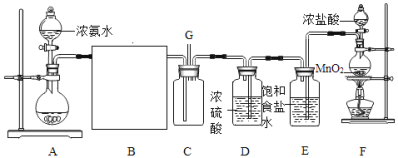
��Ŀ����
����Ŀ����֪�缫��ÿͨ��96500 C�ĵ����ͻ���1 mol���ӷ���ת�ơ���ȷ�������������ڶ��Ե缫���ԶƲ���ʽ�����Ľ�������������ȷ����������ͨ�����صĵ�����ʵ�ʲ����У������������ƣ���ͼ��ʾ������˵������ȷ����
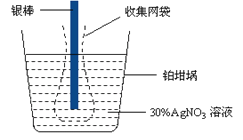
A. ��Ҫ�ⶨ��ⱥ��ʳ��ˮʱͨ���ĵ������ɽ������������е������������ص��������������������Դ�ĸ���������
B. �������ǰ�������������仯���ý������ij�����Ϊ108.0 mg�����������ͨ�����صĵ���Ϊ96.5 C
C. ʵ���У�Ϊ�˱������ܽ�����п��ܲ����Ľ����������������������²������������缫��������һ���ռ���������û���ռ����������������ƫ�ߡ�
D. �������е�����Ӧ���Դ�����������������������ĵ缫��Ӧ�ǣ�Ag+ + e- = Ag
���𰸡�A
���������õ������൱�ڲ��϶��������Ը����������е������������ص��������������������Դ�ĸ�����������A��������Ag�����ʵ���Ϊ![]() ������Ag++e-=Ag��֪ͨ���ĵ������ʵ���Ϊ0.001mol��ͨ������Ϊ96.5C����B��ȷ�����ܽ�ʱ��Щ��ԭ�ӿ���δʧ���ӱ�������ӣ�����ֱ�ӵ��䵽�������У���ɲ��������ؽ϶࣬���¼�����ĵ���ƫ�����Ա��������ռ�������C��ȷ���õ������൱�ڲ��϶�����������Pt����Ag++e-�TAg����D��ȷ��
������Ag++e-=Ag��֪ͨ���ĵ������ʵ���Ϊ0.001mol��ͨ������Ϊ96.5C����B��ȷ�����ܽ�ʱ��Щ��ԭ�ӿ���δʧ���ӱ�������ӣ�����ֱ�ӵ��䵽�������У���ɲ��������ؽ϶࣬���¼�����ĵ���ƫ�����Ա��������ռ�������C��ȷ���õ������൱�ڲ��϶�����������Pt����Ag++e-�TAg����D��ȷ��