��Ŀ����
9����ҵ������ʱ�����ô�������Ӧ��SO2ת��ΪSO3��һ���Ĺؼ����裮��1���ڱ�״���£���a L��SO2��Cl2��ɵĻ������ͨ��200mL 0.1mol/L��Fe2��SO4��3��Һ�У���ַ�Ӧ����Һ���ػ�ɫ��dz����Ӧ�����Һ�м���������BaCl2��Һ�������ó������ˡ�ϴ�ӡ��������أ�������Ϊ23.3g������������SO2�����Ϊ0.896L��a��ȡֵ��ΧΪ��1.344��a��1.792��
��2��ij�¶��£�SO2��g��+$\frac{1}{2}$O2��g��?SO3��g����H=-98kJ•mol-1����ʼʱ��100L���ܱ������м���4.0
mol SO2��g����10.0mol O2��g��������Ӧ�ﵽƽ��ʱ���ų�����196kJ�����¶���ƽ�ⳣ��K=3.33��
��3��һ�������£���һ���������ܱ������г���2mol SO2��1mol O2���������з�Ӧ��
2SO2��g��+O2��g��?2SO3��g�����ﵽƽ���ı�����������SO2��O2��SO3����ƽ��Ũ�ȶ���ԭ���������ACF������ĸ����
A�������¶Ⱥ�����������䣬����2mol SO3
B�������¶Ⱥ�����������䣬����2mol N2
C�������¶Ⱥ�����������䣬����0.5mol SO2��0.25mol O2
D�������¶Ⱥ�������ѹǿ���䣬����1mol SO3
E�������¶�
F���ƶ�����ѹ������
��4������ʱ��BaSO4��Ksp=1.08��10-10���ֽ��������BaCl2��Һ��2.0��10-3mol/L��Na2SO4��Һ��ϣ���Ҫ����BaSO4������BaCl2��Һ����СŨ��Ϊ2.16��10-7mol/L��
��5��N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע����һ���¶��£��ں����ܱ�������N2O5�ɷ������з�Ӧ��2N2O5��g��?4NO2��g��+O2��g����H��0��
���Ϊ��Ӧ��T1�¶��µIJ���ʵ�����ݣ�
| t/s | 0 | 50 | 100 |
| c��N2O5��/mol•L-1 | 5.0 | 3.5 | 2.4 |
������H2��O2��������Na2CO3��ɵ�ȼ�ϵ�أ����õ�ⷨ�Ʊ�N2O5��װ����ͼ��ʾ������YΪCO2��
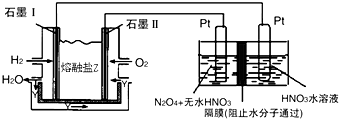
д��ʯīI�缫�Ϸ�����Ӧ�ĵ缫��ӦʽH2+CO32--2e-=CO2+H2O��
���� ��1��SO2��Cl2��ɵĻ������ͨ��Fe2��SO4��3��Һ�У�����������������ǿ�������ӣ������������������Ӧ��SO2+Cl2+2H2O=2HCl+H2SO4����ַ�Ӧ����Һ���ػ�ɫ��dz��˵��������������������2Fe3++SO2+H2O=SO42-+2Fe2++2H+������������ȫ��Ӧ����Ӧ�����Һ������BaCl2��Һ�����ɵ�23.3g����ΪBaSO4��������Ԫ���غ��֪n��BaSO4��=3n[Fe2��SO4��3]+n��SO2�����ݴ˼���n��SO2���������Ƕ������������
�����������������������ݷ���ʽ��֪n��Cl2����n��SO2��������������ȫ����������������ʱ�������ʵ������������������ݷ�Ӧ2Fe3++SO2+H2O=SO42-+2Fe2++2H+��֪��0.04mol�������������0.02mol��������ʣ���0.02mol��������Ϊ������������ʱ������������С��
��2�����ݷ�Ӧ�ȼ��㷴Ӧ�Ķ��������ټ���ƽ��ʱ��������ʵ���������K=$\frac{c��S{O}_{3}��}{c��S{O}_{2}����\sqrt{c��{O}_{2}��}}$���㣻
��3��A�������¶Ⱥ�����������䣬����2mol SO3��ƽ�����淴Ӧ�ƶ�����SO2��O2��SO3����ƽ��Ũ�ȶ���ԭ������
B�������¶Ⱥ�����������䣬����2mol N2����Ӧ���������Ũ�Ȳ��䣬ƽ�ⲻ�ƶ���
C�������¶Ⱥ�����������䣬����0.5mol SO2��0.25mol O2��ƽ��������Ӧ�����ƶ�����SO2��O2��SO3����ƽ��Ũ�ȶ���ԭ������
D�������¶Ⱥ�������ѹǿ���䣬�ٳ���1mol SO3����ЧΪ��ʼ����3mol SO2��1.5mol O2��Ϊ��Чƽ�⣬ƽ��ʱ��ͬ���ʵ�Ũ����ȣ�
E�������¶ȣ�ƽ�����淴Ӧ�ƶ���
F���ƶ�����ѹ�����壬�����С�������ʵ�Ũ�ȶ�����
��4��Na2SO4��Һ��Ũ��Ϊ2��10-3mol/L���������Ϻ���Һ��c��CO32-��=1��10-3mol/L������Ksp=c��CO32-��•c��Ba2+����������Һ��c��Ba2+����ԭ��ҺBaCl2��Һ����СŨ��Ϊ�����Һ��c��Ba2+����2����
��5���ٸ���v=$\frac{��c}{��t}$����v��N2O5�����ٸ�������֮�ȵ��ڻ�ѧ������֮�ȼ���v��NO2����
�ڱ���������ȼ������ˮ��ʯīIΪ��������ͼ��֪������ʧȥ���ӣ���̼������ӷ�Ӧ���ɶ�����̼��ˮ��
��� �⣺��1��SO2��Cl2��ɵĻ������ͨ��Fe2��SO4��3��Һ�У�����������������ǿ�������ӣ������������������Ӧ��SO2+Cl2+2H2O=2HCl+H2SO4����ַ�Ӧ����Һ���ػ�ɫ��dz��˵��������������������2Fe3++SO2+H2O=SO42-+2Fe2++2H+������������ȫ��Ӧ����Ӧ�����Һ������BaCl2��Һ�����ɵ�23.3g����ΪBaSO4�������ʵ���=$\frac{23.3g}{233g/mol}$=0.1mol��
������Ԫ���غ��֪n��BaSO4��=3n[Fe2��SO4��3]+n��SO2������n��SO2��=0.1mol-3��0.2L��0.1mol•L-1=0.04mol��V��SO2��=0.04mol��22.4L/mol=0.896L��
�����������������������ݷ���ʽ��֪n��Cl2����n��SO2��������������ȫ����������������ʱ�������ʵ������n��Cl2����0.04mol���ʻ������������Ϊ����0.04mol+0.04mol����22.4L/mol=1.792L�����ݷ�Ӧ2Fe3++SO2+H2O=SO42-+2Fe2++2H+��֪��0.04mol�������������0.02mol��������ʣ���0.02mol����������Ҫ0.02mol�����������������������ʵ�������Ϊ0.02mol������������С���Ϊ����0.04mol+0.02mol����22.4L/mol=1.344L����a��ȡֵ��ΧΪ1.344��a��1.792��
�ʴ�Ϊ��0.896��1.344��a��1.792��
��2��ij�¶��£�SO2��g��+$\frac{1}{2}$O2��g��?SO3��g����H=-98kJ•mol-1����ʼʱ��100L���ܱ������м���4.0
mol SO2��g����10.0mol O2��g��������Ӧ�ﵽƽ��ʱ���ų�����196kJ���μӷ�Ӧ��SO2��g��Ϊ2mol����
SO2��g��+$\frac{1}{2}$O2��g��?SO3��g��
��ʼ����mol����4 10 0
�仯����mol����2 1 2
ƽ������mol����2 9 2
ƽ�ⳣ��K=$\frac{c��S{O}_{3}��}{c��S{O}_{2}����\sqrt{c��{O}_{2}��}}$=$\frac{\frac{2}{100}}{\frac{2}{100}��\sqrt{\frac{9}{100}}}$=3.33��
�ʴ�Ϊ��3.33��
��3��A�������¶Ⱥ�����������䣬����2mol SO3��ƽ�����淴Ӧ�ƶ���ƽ�ⳣ�����䣬��SO2��O2��SO3����ƽ��Ũ�ȶ���ԭ������A��ȷ��
B�������¶Ⱥ�����������䣬����2mol N2����Ӧ���������Ũ�Ȳ��䣬ƽ�ⲻ�ƶ�����B����
C�������¶Ⱥ�����������䣬����0.5mol SO2��0.25mol O2��ƽ��������Ӧ�����ƶ�����SO2��O2��SO3����ƽ��Ũ�ȶ���ԭ������C��ȷ��
D�������¶Ⱥ�������ѹǿ���䣬�ٳ���1mol SO3����ЧΪ��ʼ����3mol SO2��1.5mol O2��Ϊ��Чƽ�⣬ƽ��ʱ��ͬ���ʵ�Ũ����ȣ���D����
E�������¶ȣ�ƽ�����淴Ӧ�ƶ���SO3��Ũ�ȼ�С����E����
F���ƶ�����ѹ�����壬�����С�������ʵ�Ũ�ȶ�����F��ȷ��
��ѡ��ACF��
��4��Na2SO4��Һ��Ũ��Ϊ2��10-3mol/L���������Ϻ���Һ��c��CO32-��=1��10-3mol/L������Ksp=c��CO32-��•c��Ba2+��=1.08��10-10��֪��c��Ba2+��=$\frac{1.08��1{0}^{-10}}{1��1{0}^{-3}}$mol/L=1.08��10-7mol/L��ԭ��ҺBaCl2��Һ����СŨ��Ϊ�����Һ��c��Ba2+����2������ԭ��ҺBaCl2��Һ����СŨ��Ϊ2��1.08��10-7mol/L=2.16��10-7mol/L��
�ʴ�Ϊ��2.16��10-7��
��5��������֮�ȵ��ڻ�ѧ������֮�ȣ���v��NO2��=2v��N2O5��=2��$\frac{��5-3.5��mol/L}{50s}$=0.06mol•L-1•s-1��
�ʴ�Ϊ��0.06mol•L-1•s-1��
�ڱ���������ȼ������ˮ��ʯīIΪ��������ͼ��֪������ʧȥ���ӣ���̼������ӷ�Ӧ���ɶ�����̼��ˮ���缫��ӦʽΪ��H2+CO32--2e-=CO2+H2O��
�ʴ�Ϊ��H2+CO32--2e-=CO2+H2O��
���� ���⿼�黯ѧƽ�������Ӱ�����ء��������㡢�ܶȻ��йؼ��㡢ԭ��صȣ��Ƕ�ѧ���ۺ������Ŀ��飬�ѶȽϴ�

 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д� ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�| A�� | 98.3%��������뵽ϡ����������Һ�У�H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ/mol | |
| B�� | ��NH4Fe��SO4��2��Һ����μ���Ba��OH��2��Һ�����ܷ����ķ�Ӧ�ǣ�3NH4++Fe3++3SO42-+3Ba2++6OH-�T3BaSO4��+Fe��OH��3��+3NH3•H2O | |
| C�� | �ữ�ĵ��۵⻯����Һ�ڿ����б�����4I-+O2+4H+�T2I2+2H2O | |
| D�� | Na2SO3��Һʹ����KMnO4��Һ��ɫ��5SO32-+6H++2MnO4-�T5SO42-+2Mn2++3H2O |
 ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�
ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�CH3CH2OH$��_{170��}^{H_{2}SO_{4}��Ũ����}$CH2�TCH2
CH2�TCH2+Br2��BrCH2CH2Br
���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140����ˮ�������ѣ�������������������Ҵ��Ƹ�1��2-���������װ����ͼ��ʾ��
�й������б����£�
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | -130 | 9 | -116 |
��1���ڴ�װ�ø�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶���ߵ�170�����ң�������ҪĿ����d��������ȷѡ��ǰ����ĸ��
a��������Ӧ b���ӿ췴Ӧ�ٶ� c����ֹ�Ҵ��ӷ�d�����ٸ�������������
��2����װ��C��Ӧ����c����Ŀ�������շ�Ӧ�п������ɵ��������壺������ȷѡ��ǰ����ĸ��
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��3���жϸ�װ�ø���Ӧ�Ѿ�������������������ɫ��ȫ��ȥ��
��4����1��2-��������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ���²㣨��ϡ������¡�����
��5�������������������������ѣ���������ķ�����ȥ��
��6����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ���DZ���������ӷ������ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ���Dz�Ʒ1��2-��������ķе�ͣ�������ȴ�����̶��������ܣ�
| A�� | ��ѧ����һ�������� | |
| B�� | ��ѧ������ʹ���Ӽ����ϣ�Ҳ����ʹԭ�Ӽ����� | |
| C�� | ��ѧ��Ӧ�����з�Ӧ�ﻯѧ�����ѣ������ﻯѧ���γ� | |
| D�� | �Ȼ��ƹ�������ˮû����������������û���ƻ���ѧ�� |
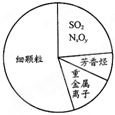 ��ͼΪ��������Ҫ�ɷ�ʾ��ͼ������˵������ȷ���ǣ�������
��ͼΪ��������Ҫ�ɷ�ʾ��ͼ������˵������ȷ���ǣ�������| A�� | �ؽ������ӿɵ��µ����ʱ��� | |
| B�� | ������ķ����� | |
| C�� | ������д����ŷ�SO2��NxOy������������⻯ѧ���� | |
| D�� | ����β���Ĵ����ŷ������������������Ϊ����֮һ |
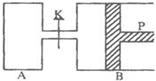 ͼ�У�PΪһ�����ɻ����Ļ������ر�K���ֱ�������A��B�и�����2molX��2molY����ʼʱ��VA=aL��VB=0.8aL����ͨ�ܵ�������Բ��ƣ�������ͬ�¶Ⱥ��д������ڵ������£��������и��Է���������Ӧ��
ͼ�У�PΪһ�����ɻ����Ļ������ر�K���ֱ�������A��B�и�����2molX��2molY����ʼʱ��VA=aL��VB=0.8aL����ͨ�ܵ�������Բ��ƣ�������ͬ�¶Ⱥ��д������ڵ������£��������и��Է���������Ӧ��