��Ŀ����
����Ŀ��ij����С���о���������Al2O3����������֪����������Ҫ�ɷ���Al2O3��������Fe2O3��SiO2����������������ȡAl2O3�Ĺ���������
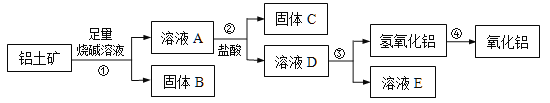
��1������B����Ҫ��;����д��1��������________________��
��2�������������������м��������ռ���Һ��������Ӧ�����ӷ���ʽ�� �� ��
�������У��������������Ļ�ѧ����ʽ�� ��
��3����ʵ����������ù��徫ȷ����������С�鷢�������������������������ԭ������������ȣ������������Al2O3������������_______��������һλС����
��4����ҵ����ȡAlCl3��Al2O3��C��Cl2�ڸ��������·�Ӧ��ÿ����0��5 mol ̼���ʣ�ת��1 mol���ӣ���Ӧ�Ļ�ѧ����ʽ�� ��
���𰸡���1������ԭ��������Ϳ�ϡ������
��2��Al2O3+2OH��= 2AlO2��+H2O SiO2+2OH��= SiO32��+ H2O
AlCl3 +3NH3��H2O = Al��OH��3��+ 3NH4Cl
��3��65��4%
��4��Al2O3+3Cl2+3C![]() 2AlCl3 +3CO
2AlCl3 +3CO
��������
�����������1������������Ҫ�ɷ���Al2O3��������Fe2O3��SiO2�������������м���NaOH��Һ������Al2O3��SiO2����NaOH��Ӧ����˹���BΪFe2O3����Ҫ��;������ԭ��������Ϳ�ϡ����������2�����������м��������ռ���Һ��Al2O3��NaOH��Ӧ����NaAlO2��SiO2��NaOH��Ӧ����Na2SiO3�����ӷ���ʽΪ��Al2O3+2OH��= 2AlO2��+H2O ��SiO2+2OH��= SiO32��+ H2O ������ҺA�м������ᣬ����CΪH2SiO3����ҺDΪAlCl3��NaCl���������У�����ҺD���백ˮ����ˮ��AlCl3��Ӧ�����������������ӷ���ʽΪ��AlCl3 +3NH3��H2O = Al��OH��3��+ 3NH4Cl����3����������������������Ϊm����ԭ�����������Ϊm������Ԫ�ص����ʵ���Ϊ��![]() ����Al2O3�����ʵ���Ϊ��
����Al2O3�����ʵ���Ϊ��![]() ����ԭ��������Al2O3������Ϊ��
����ԭ��������Al2O3������Ϊ��![]() ������������Al2O3��������������
������������Al2O3��������������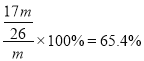 ����4��ÿ����0��5 mol ̼���ʣ�ת��1 mol���ӣ���̼Ԫ�ػ��ϼ���0������Ϊ+2�ۣ�������ɵIJ�����CO��AlCl3 ���ʷ�Ӧ�Ļ�ѧ����ʽΪ��Al2O3+3Cl2+3C
����4��ÿ����0��5 mol ̼���ʣ�ת��1 mol���ӣ���̼Ԫ�ػ��ϼ���0������Ϊ+2�ۣ�������ɵIJ�����CO��AlCl3 ���ʷ�Ӧ�Ļ�ѧ����ʽΪ��Al2O3+3Cl2+3C ![]() 2AlCl3 +3CO��
2AlCl3 +3CO��



