��Ŀ����
3�� ��500���£���ij�����ܱ�������Ͷ��amolA2��bmolB2�������淴ӦA2��g��+B2��g��?2AB��g������H��0��AB�����ʵ�����ʱ���ϵ��ͼ��ʾ��
��500���£���ij�����ܱ�������Ͷ��amolA2��bmolB2�������淴ӦA2��g��+B2��g��?2AB��g������H��0��AB�����ʵ�����ʱ���ϵ��ͼ��ʾ����1���ﵽƽ��ʱ��A2��B2��ת����֮��Ϊb��a������ͼ���������ݣ��ж��ܷ����ƽ�ⳣ��K���������ɣ����ܣ�ƽ�ⳣ������ʽK=$\frac{{c}^{2}��AB��}{c��{A}_{2}����c��{B}_{2}��}$����Ӧǰ�������������䣬���������ʵ�������Ũ�ȼ���ƽ�ⳣ������ƽ��ʱ����ֵ����ʵ�����ת�����йأ�
��2�����ﵽƽ���ͬʱ������������������¶ȣ�ƽ���ƶ�������b��
a������b������c������d����ȷ��
��3�����ﵽƽ��ʱ���������г���1molAB��g����A2�����ʵ����������������С�����䡱����
���� ��1����500���£���ij�����ܱ�������Ͷ��amolA2��bmolB2��ƽ��ʱAB�����ʵ���Ϊm mol����
A2��g��+B2��g��?2AB��g��
��ʼ����mol����a b 0
�仯����mol����0.5m 0.5m m
ƽ������mol����a-0.5m b-0.5m m
�ݴ˼���ת����֮�ȣ�ƽ�ⳣ������ʽK=$\frac{{c}^{2}��AB��}{c��{A}_{2}����c��{B}_{2}��}$����Ӧǰ�������������䣬���������ʵ�������Ũ�ȼ���ƽ�ⳣ������ƽ��ʱ����ֵ����ʵ�����ת�����йأ�
��2�����ﵽƽ���ͬʱ������������������¶ȣ�����������ѹǿ��С����Ӱ��ƽ���ƶ����������¶�ƽ�������ȷ�Ӧ�ƶ���
��3�����ﵽƽ��ʱ���������г���1molAB��g����ƽ�������ƶ���
��� �⣺��1����500���£���ij�����ܱ�������Ͷ��amolA2��bmolB2��ƽ��ʱAB�����ʵ���Ϊm mol����
A2��g��+B2��g��?2AB��g��
��ʼ����mol����a b 0
�仯����mol����0.5m 0.5m m
ƽ������mol����a-0.5m b-0.5m m
�ﵽƽ��ʱ��A2��B2��ת����֮��Ϊ$\frac{0.5m}{a}$��$\frac{0.5m}{b}$=b��a��ƽ�ⳣ������ʽK=$\frac{{c}^{2}��AB��}{c��{A}_{2}����c��{B}_{2}��}$����Ӧǰ�������������䣬���������ʵ�������Ũ�ȼ���ƽ�ⳣ������ƽ��ʱ����ֵ����ʵ�����ת�����йأ��������ƽ�ⳣ����
�ʴ�Ϊ��b��a�����ܣ�ƽ�ⳣ������ʽK=$\frac{{c}^{2}��AB��}{c��{A}_{2}����c��{B}_{2}��}$����Ӧǰ�������������䣬���������ʵ�������Ũ�ȼ���ƽ�ⳣ������ƽ��ʱ����ֵ����ʵ�����ת�����йأ�
��2�����ﵽƽ���ͬʱ������������������¶ȣ�����������ѹǿ��С����Ӱ��ƽ���ƶ�������ӦΪ���ȷ�Ӧ���������¶�ƽ�������ƶ�����ѡ��b��
��3�����ﵽƽ��ʱ���������г���1molAB��g����ƽ�������ƶ���A2�����ʵ��������ʴ�Ϊ������
���� ���⿼�黯ѧƽ�������Ӱ�����ء�ƽ�ⳣ����ע���ƽ�ⳣ�������������Ӧ�ã��Ѷ��еȣ�

| A�� | SO2��H2S��O2 | B�� | CH4��H2��O2 | C�� | C2H4��N2��NH3 | D�� | HCl��C12��CO2 |
| A�� | O2��O3 | B�� | H2��D2 | C�� | ${\;}_{1}^{1}$H��${\;}_{1}^{2}$H | D�� | CH4 ��C2H6 |
| A�� |  | B�� | 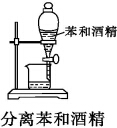 | C�� |  | D�� | 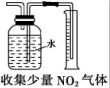 |
 A��B��C����ǿ����ʣ�������ˮ�е���������������ʾ��
A��B��C����ǿ����ʣ�������ˮ�е���������������ʾ��| ������ | Ag+������Na+ |
| ������ | NO3-����SO42-������Cl- |
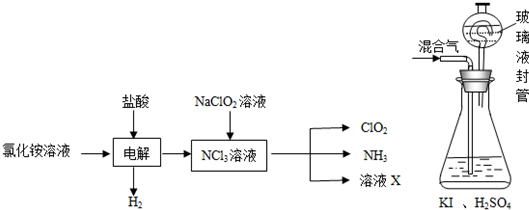
��1��MΪ��Դ�ĸ������������������e�缫�ϲ������������Ϊ2.8L����״���£���
��2��e�缫�Ϸ����ĵ缫��ӦΪ2H++2e-=H2����
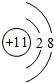 ��
��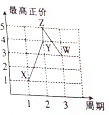 X��Y��Z��W��V�����ֶ���������Ԫ�أ�����Ԫ�ص�����������������۵Ĺ�ϵ��ͼ��ʾ����֪VԪ�ص�ԭ����������һ����ͬ��Ԫ�ص�ԭ��������һ�룮
X��Y��Z��W��V�����ֶ���������Ԫ�أ�����Ԫ�ص�����������������۵Ĺ�ϵ��ͼ��ʾ����֪VԪ�ص�ԭ����������һ����ͬ��Ԫ�ص�ԭ��������һ�룮