��Ŀ����
13����֪�ϳɰ���ӦN2��g��+3H2��g��?2NH3��g����H��0����1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��K=$\frac{{c}^{2}��N{H}_{3}��}{c��{N}_{2}��•{c}^{3}��{H}_{2}��}$��
��2���÷�Ӧ�Ļ�ѧƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���ʾ��
| T/K | 473 | 573 | 673 | �� |
| K | 4.4��10-2 | K1 | K2 | �� |
��3���ϳɰ���ũҵ�������к���Ҫ�����壬��ʵ�ʹ�ҵ�����У����������д�ʩ�����п�������������ԭ�����͵���BD������ĸ����
A��������ý�ӿ컯ѧ��Ӧ���� B�����ýϸ�ѹǿ��20MPa��50MPa��
C�����ýϸ��¶ȣ�400�桫500�棩 D�������ɵİ�Һ������ʱ����ϵ�з��������
���� ��1����ѧƽ�ⳣ��=$\frac{������ƽ��Ũ��ϵ���η��ij˻�}{��Ӧ��ƽ��Ũ��ϵ���η��ij˻�}$�����ݻ�ѧƽ�ⳣ������ʽ�ĺ�������д��
��2�����ڷ��ȷ�Ӧ���¶�Խ�ߣ�KԽС��
��3����������ԭ��Ϊ������ı�Ӱ��ƽ�������֮һ��ƽ�⽫�����ܹ��������ָı�ķ����ƶ���ʹ����������ԭ��ʱ���÷�Ӧ�����ǿ��淴Ӧ��������������ԭ�������ã�
��� �⣺��1�����ݻ�ѧƽ�ⳣ��=$\frac{������ƽ��Ũ��ϵ���η��ij˻�}{��Ӧ��ƽ��Ũ��ϵ���η��ij˻�}$��֪���÷�Ӧ��ƽ�ⳣ��K=$\frac{{c}^{2}��N{H}_{3}��}{c��{N}_{2}��•{c}^{3}��{H}_{2}��}$��
�ʴ�Ϊ��$\frac{{c}^{2}��N{H}_{3}��}{c��{N}_{2}��•{c}^{3}��{H}_{2}��}$��
��2���ϳɰ���ӦN2��g��+3H2��g��?2NH3��g����H��0�����ڷ��ȷ�Ӧ���¶�Խ�ߣ�KԽС������K1��K2��
�ʴ�Ϊ������
��3��A��������ý�ӿ컯ѧ��Ӧ���ʣ�����������ý���������ͷ�Ӧ�Ļ�ܣ��ӿ췴Ӧ���ʣ����ı仯ѧƽ�⣬��������������ԭ�����ͣ���A�����ϣ�
B����Ӧ�����������С�ķ�Ӧ����ѹƽ��������з��ϻ�ѧƽ���ƶ�ԭ������������������ԭ�����ͣ���B���ϣ�
C���ϳɰ���ӦΪ���ȷ�Ӧ�������¶Ȳ�����ƽ�����������ƶ���������ȴ����߷�Ӧ���ʣ��������Դ�����ɳ����ԭ����������C�����ϣ�
D�������ɵİ�Һ������ʱ����ϵ�з������������������Ũ�ȣ�ƽ��������У���������������ԭ�����ͣ���D���ϣ�
��ѡBD��
���� ���⿼��ѧ��ƽ�ⳣ������д�Լ��¶ȶ�ƽ�ⳣ����Ӱ��֪ʶ�����ڽ̲�֪ʶ�Ŀ��飬�ѶȲ���

| A�� | ����ˮ�������CO2��H2O | B�� | ��֬ˮ������а����� | ||
| C�� | ��������ǻ�Ϊͬ���칹�� | D�� | ��������������ҪӪ������ |
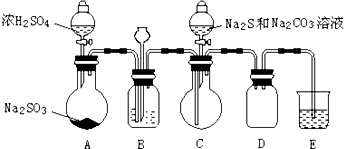
��ʵ��һ����������ƣ�Na2S2O3������ȡʵ�����Ʊ�װ����ͼ��ʾ���г�װ����ʡ�ԣ���
�ش����⣺
��1����װ��B�еij���©����������ƽ��ѹǿ��
��B�����ʢװ��Һ����c��
a������ˮ b������Na2SO3��Һ c������NaHSO3��Һ d������NaHCO3��Һ
��2����װ��C����Na2S2O3���ɣ�
��д��װ��C�з�Ӧ�Ļ�ѧ����ʽ��2Na2S+Na2CO3+4SO2�T3Na2S2O3+CO2
�ڽ�����Ӧ��ȡC����Һ��������Ũ������ȴ�ᾧ������ϴ�ӡ�����õ�Na2S2O3•5H2O��
��3��װ��E��ΪNaOH��Һ��
��ʵ�������������ƣ�Na2S2O3��������
��4��Na2S2O3��������Һ�в����ȶ����ڣ��������ӷ���ʽ��ʾ�ù��̣�2H++S2O32-=SO2��+S��+H2O
��5����μ���Na2S2O3�����ڿ������ѱ�����������Na2S2O3�������Թ��У�������ˮ�ܽ⣬�μ��������Ȼ�����Һ�����а�ɫ�������ɣ�˵���ѱ�������
��ʵ��������Na2S2O3�ⶨ��ˮ�е�Ba2+
��6����ҵ�ϳ���Na2S2O3��Һ�ⶨ��ˮ��Ba2+��Ũ�ȣ��������£�ȡ��ˮ25.00mL�������ʵ�����ȼ������� K2Cr2O7��Һ����BaCrO4���������ˡ�ϴ�Ӻ�������ϡ�����ܽ⣬��ʱCrO42-ȫ��ת��ΪCr2O72-���ټӹ�KI��Һ����ַ�Ӧ��û����ҺV mL������ƽ���ֳ�4�ȷݣ����������Һ��ָʾ������0.001 00mol•L-1 ��Na2S2O3��Һ���еζ�����Ӧ��ȫʱ��������ݼ�¼�����ʾ��
| ʵ���� | 1 | 2 | 3 | 4 |
| ����Na2S2O3�� ��Һ�����/mL | 18.02 | 17.98 | 18.00 | 20.03 |
Cr2O72-+6I-+14H+�T2Cr3++3I2+7H2O��
I2+2S2O32-�T2I-+S4O64-��
���жϴﵽ�ζ��յ�������ǵμ����һ��Na2S2O3��Һʱ��Һ����ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ��
�ڼ����ˮ��Ba2+�����ʵ���Ũ��9.6��10-4mol•L-1��
| A�� | ��E��F��W�γɵĻ�����EFW2����E��W�����γɻ�����E2W2 | |
| B�� | F������������Ӧ��ˮ����һ����������������Һ��Ӧ | |
| C�� | �ȶ��ԣ�Z���⻯�W���⻯�� | |
| D�� | �е㣺Z���⻯�W���⻯�� |
| A�� | 2H2������+O2�������T2H2O��������H1 2H2������+O2�������T2H2O��Һ����H2 | |
| B�� | S������+O2�������TSO2��������H1 S���̣�+O2�������TSO2��������H2 | |
| C�� | C���̣�+O2�������TCO2��������H1 C���̣�+$\frac{1}{2}$O2 �������TCO��������H2 | |
| D�� | H2������+Cl2�������T2HCl��������H1 $\frac{1}{2}$H2������+$\frac{1}{2}$Cl2�������THCl��������H2 |
| A�� | ���Ҵ����͡��Ĺ㷺ʹ������Ч�����к�������ŷ� | |
| B�� | ��ʳ�ؽ����ζ�������ж������ȷ����Ƿ��ô����Ķ�����ţ�� | |
| C�� | �������䡱ȡ�����������䡱��������ı���ɡһ�������������� | |
| D�� | ����ˮ���������Խ������ˮ��ӦΣ��������ˮ�м��˾�ˮ����������ʹ��ˮ���� |
| A�� | ��-���屽ֻ��һ�� | |
| B�� | ������������ԭ����ͬһƽ���� | |
| C�� | ���ױ�û��ͬ���칹�� | |
| D�� | �����ܷ����ӳɷ�ӦҲ�ܷ���ȡ����Ӧ |