��Ŀ����
����Ŀ��
A����500 mL����ƿ������500 mL�ռ���Һ |
B������Һ�ܣ����ʽ�ζ��ܣ���ȡ50.00 mL�ռ���Һ����ƿ�в��Ӽ��μ���ָʾ�� |
C������ƽ��ȷ��ȡ�ռ���Ʒm g�����ձ��м�����ˮ�ܽ� |
D�������ʵ���Ũ��Ϊc mol/L�ı�H2SO4��Һװ����ʽ�ζ��ܣ�����Һ�棬���¿�ʼ�̶�V1mL |
E������ƿ�µ�һ�Ű�ֽ���ζ����յ㣬��¼�յ�̶�ΪV2mL ������������⣺
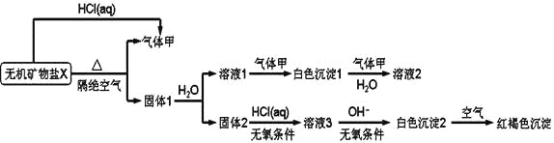
��1����ȷ�IJ��������ǣ���д��ĸ��________��________��________��D��________.
��2���ζ��ܶ���Ӧע��_______________________.
��3����������ƿ�µ�һ�Ű�ֽ��������___________________.
��4������D��Һ��Ӧ������________________________�����첿��Ӧ____________��
��5���ζ����յ�ʱ��ƿ����Һ��pHԼΪ___________���յ�ʱ����ɫ�仯��________��
��6������ʽ�ζ���û�ñ�H2SO4��ϴ����Բⶨ����к�Ӱ��________������ƫ��������ƫ����������Ӱ������������������ȷ����
��7�����ռ���Ʒ�Ĵ��ȼ���ʽ��__________________��
���𰸡���1��C A B E ��2���ζ��ܴ�ֱ��װҺ�ͷ�Һ����Ҫ��һ�ᣬ��Һ�����²������仯ʱ���ܶ���������ʱҪƽ�ӣ������밼Һ����͵���ƽ������Ӧ��0.01 mL ��3������ȷ�жϵζ��յ�ʱ��Һ����ɫ�仯��� ��4����̶Ȼ���̶����µ�ijһ�̶� ������Һ��������
��5��3.1��4.4 �ɻ�ɫ��Ϊ��ɫ��6��ƫ�ߣ�7��![]()
�������������������1��ʵ��ʱӦ�ȳ���һ�������Ĺ��壬�ܽ�����Ƴ���Һ����ȡ����Һ����ƿ�У�Ȼ���ñ�Һ���еζ�������ȷ�IJ���������C��A��B��D��E��
��2���ζ��ܴ�ֱ��Һ�治�ٱ仯ʱ�ſɶ�������������Һ��Һ����͵���ƽ���ζ��ܶ���Ӧ������0.01ml��
��3������ƿ�µ�һ�Ű�ֽʹ�ζ��յ���ɫ�仯�����ԣ�����ȷ�жϵζ��յ�ʱ��Һ����ɫ�仯�����
��4���ζ���0�̶����ϣ��ζ�ǰӦ���ڵ���̶Ȼ������µ�ijһ�̶ȣ�Ϊ��С�����첿��Ӧ����Һ�壬�����ݣ�
��5��ָʾ��Ϊ���ȣ���ɫ��ΧΪ3.1��4.4���յ�ʱpHԼΪ4����Һ�ɻ�ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ�Ϊԭ������ɫ��
��6�����ҺŨ�ȱ�С��������ⶨ���Ϊƫ�ߣ�
��7���ε����ĵ�����Ϊ��n�����ᣩ=cV=��V2-V1����10-3L �� c mol/L�����ݷ�Ӧ���̿�֪��n��NaOH��=2n�����ᣩ=2c��V2-V1����10-3mol������ԭ����Ʒ���������Ƶ����ʵ���Ϊ��2c��V2-V1����10-3mol��![]() =2c��V2-V1����10-2mol������Ʒ���������Ƶ�����Ϊm��NaOH��=nM=80c��V2-V1����10-2g������ռ���Ʒ�Ĵ���Ϊ:
=2c��V2-V1����10-2mol������Ʒ���������Ƶ�����Ϊm��NaOH��=nM=80c��V2-V1����10-2g������ռ���Ʒ�Ĵ���Ϊ:![]() ��100% =
��100% =![]() ��
��