��Ŀ����
8��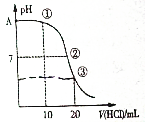 �����£���0.1000mol•L-1 HCl��Һ�ζ�20.00mL 0.1000mol•L-1 NH3•H2O��Һ���ζ�������ͼ������˵����ȷ���ǣ�������
�����£���0.1000mol•L-1 HCl��Һ�ζ�20.00mL 0.1000mol•L-1 NH3•H2O��Һ���ζ�������ͼ������˵����ȷ���ǣ�������| A�� | A���pH=13 | |
| B�� | ����Һ��c��NH4+����c��Cl-����c��OH-��=c��H+�� | |
| C�� | ����Һ����ʱ��Һ�е�ˮ�����c��H+����ԭ��ˮ�е����c��H+��С | |
| D�� | �ζ������п��ܳ��֣�c��NH3•H2O����c��NH4+����c��OH-����c��Cl-����c��H+�� |
���� A��һˮ�ϰ�Ϊ������ʣ�0.1000mol•L-1 NH3•H2O��Һ������������Ũ��С��0.1000mol•L-1��
B������Һ��ʾ���ԣ�������Һ�еĵ���غ�������ش�
C��20mL0.1000mol/L�����20.00mL0.1000mol/LNH3•H2O��Һ��Ӧ���õ������Ȼ����Һ��笠����ӻᷢ��ˮ�⣻
D���ζ������п��ܳ�����������ڰ�ˮ���������㡢ǡ�÷�Ӧ��������ݴ˻ش��жϣ�
��� �⣺A��һˮ�ϰ�Ϊ������ʣ�0.1000mol•L-1 NH3•H2O��Һ������������Ũ��С��0.1000mol•L-1��pH��13����A����
B������Һ��ʾ���ԣ�������Һ�еĵ���غ㣬�õ���c��NH4+��=c��Cl-����c��OH-��=c��H��+����B����
C������20mL0.1000mol/L�����20.00mL0.1000mol/LNH3•H2O��Һ��Ӧ���õ������Ȼ����Һ��笠�����ˮ��ٽ�ˮ�ĵ��룬��ʱ��Һ�е�ˮ�����c��H+����ԭ��ˮ�е����c��H+����C����
D���ζ������п��ܳ�����������ڰ�ˮ���������㡢ǡ�÷�Ӧ����������ܳ��֣�c��NH3•H2O����c��NH4+����c��OH-����c��Cl-����c��H+����������ʣ������İ�ˮ�Լ������������Ȼ��ʱ���������D��ȷ��
��ѡD��
���� ���⿼��ѧ�����֮��ķ�Ӧ����Լ���Һ������Ũ�ȵĴ�С��ϵ�Ƚ�֪ʶ�������ۺ�֪ʶ�Ŀ��飬�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�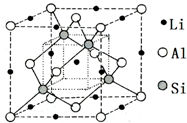
| A�� | �û����ﻯѧʽ�ɱ�ʾΪLiAlSi | |
| B�� | ������Al��Li����CsCl�Ǽ� | |
| C�� | ������Al��Si���ɽ��ʯ�Ǽ� | |
| D�� | ��������ÿ��Al���������LiΪ4�� |
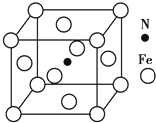 �����仯�������л���ѧ��Ӧ�ù㷺�������л��ϳ��У������������Ὣ������-NO2����ԭΪ������-NH2�����ڱ��������Ӧ�����廯����������
�����仯�������л���ѧ��Ӧ�ù㷺�������л��ϳ��У������������Ὣ������-NO2����ԭΪ������-NH2�����ڱ��������Ӧ�����廯���������� ��Fe��̬ԭ�Ӻ�������Ų�ʽΪ[Ar]3d64s2��
��Fe��̬ԭ�Ӻ�������Ų�ʽΪ[Ar]3d64s2��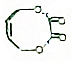 ��д����ϳ�·��ͼ��
��д����ϳ�·��ͼ��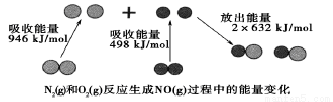
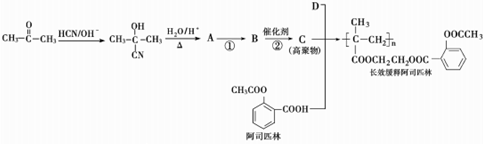
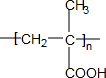 ��
��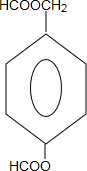 ��ֻдһ�֣���
��ֻдһ�֣���