��Ŀ����
����Ŀ�������л���A��B���Ի��ܣ��й��������£�
����ܶ�(20��) | �۵� | �е� | �ܽ��� | |
A | 0.7893 | -117.3�� | 78.5�� | ��ˮ������Ȼ��� |
B | 0.7137 | -116.6�� | 34.5�� | ������ˮ |
��1����Ҫ��ȥA��B�Ļ������������B���ɲ���_______(�����)�������ɵõ�A��
a���ؽᾧ b������ c����ȡ d����ˮ�������Һ
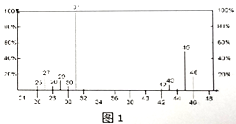
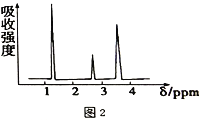
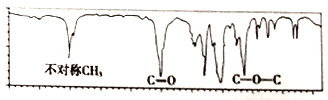
��2�����л���A�����������г��ȼ�գ�ʵ���ã�����5.4gH2O��8.8gCO2����������6.72L(��״����)��������ʵ����ʽΪ_________����Ҫȷ�������ʽ���Ƿ��������������_______(����������������)����֪�л���A�����ס��˴Ź���������ͼ1��ʾ����A�Ľṹ��ʽΪ________��
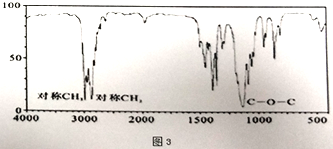
��3��������ͼ2��ʾB����Է�������Ϊ74,���������ͼ3��ʾ����B�Ľṹ��ʽΪ_________��������ŵ�����Ϊ_________��
��4��ȷ��ȡһ��������A��B�Ļ��������������г��ȼ�գ�����������ͨ����������ˮ�Ȼ��ƺͼ�ʯ�ң����������ֱ�����19.8g��35.2g������������A��B�����ʵ���֮��_________��
���𰸡� b C2H6O �� CH3CH2OH CH3CH2OCH2CH3 �Ѽ� 2��1
����������1���ɱ����е���Ϣ��֪��A��B�����л��ﻥ�ܣ����е㲻ͬ����ѡ�������룬��ѡb����2�����������غ㶨�ɵã�������������CԪ������Ϊ��8.8g��![]() =2.4g�� ������������HԪ������Ϊ��5.4g��
=2.4g�� ������������HԪ������Ϊ��5.4g��![]() =0.6g��������̼��ˮ�е���Ԫ������֮��Ϊ ��8.8g-2.4g��+��5.4g-0.6g��=11.2g��������������Ϊ
=0.6g��������̼��ˮ�е���Ԫ������֮��Ϊ ��8.8g-2.4g��+��5.4g-0.6g��=11.2g��������������Ϊ![]() ��32g/mol=9.6g�������л�������Ԫ������Ϊ11.2g-9.6g=1.6g��n��C����n��H����n��O��=
��32g/mol=9.6g�������л�������Ԫ������Ϊ11.2g-9.6g=1.6g��n��C����n��H����n��O��= ![]() ��
�� ![]() ��
�� ![]() =2��6��1�����Ի������ʵ��ʽ�����ʽ����C2H6O�����ڷ�����̼��ԭ�ӵĸ�����1:3���Ѿ��ﵽ���ͣ�������Ҫȷ�������ʽ�����������������������ʽ���Ƿ���ʽ������ʽΪC2H6O���˴Ź������ױ���������������ֻ�ѧ������ͬ����ԭ�ӣ�ǿ��֮��Ϊ3�U2�U1����A�Ľṹ��ʽΪCH3CH2OH����3��A����Է�������Ϊ74�����ݺ�����ף�A�д��ڶԳƵļ����Ѽ���ʣ���ʽ��Ϊ74-16-15��2=28����2��CH2�����ݶԳ��ԣ�A�Ľṹ��ʽΪCH3CH2OCH2CH3��������Ϊ�Ѽ�����4��A��B�Ļ�ѧʽ�ֱ�ΪC2H6O��C4H10O����C2H6O��C4H10O�����ʵ����ֱ��� x��y����
=2��6��1�����Ի������ʵ��ʽ�����ʽ����C2H6O�����ڷ�����̼��ԭ�ӵĸ�����1:3���Ѿ��ﵽ���ͣ�������Ҫȷ�������ʽ�����������������������ʽ���Ƿ���ʽ������ʽΪC2H6O���˴Ź������ױ���������������ֻ�ѧ������ͬ����ԭ�ӣ�ǿ��֮��Ϊ3�U2�U1����A�Ľṹ��ʽΪCH3CH2OH����3��A����Է�������Ϊ74�����ݺ�����ף�A�д��ڶԳƵļ����Ѽ���ʣ���ʽ��Ϊ74-16-15��2=28����2��CH2�����ݶԳ��ԣ�A�Ľṹ��ʽΪCH3CH2OCH2CH3��������Ϊ�Ѽ�����4��A��B�Ļ�ѧʽ�ֱ�ΪC2H6O��C4H10O����C2H6O��C4H10O�����ʵ����ֱ��� x��y����
C2H6O + 3 O2��2 CO2 + 3 H2O
x 2x 3x
C4H10O + 6 O2��4 CO2 + 5 H2O
y 4y 5y
3x+5y= ![]()
2x+4y =![]()
���x =0.2mol��y = 0.1 mol�������ʵ���֮��2��1��

����Ŀ�����и����е����ʣ���������ͼ����Ӱ���ֹ�ϵ��
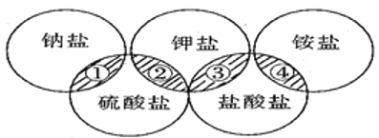
ѡ�� | �� | �� | �� | �� |
A | NaCl | K2SO4 | KCl | ��NH4��2SO4 |
B | NaCl | K2SO4 | KCl | NH4Cl |
C | Na2SO4 | K2SO4 | KCl | NH4Cl |
D | Na2SO4 | K2SO4 | KCl | ��NH4��2SO4 |
A. A B. B C. C D. D