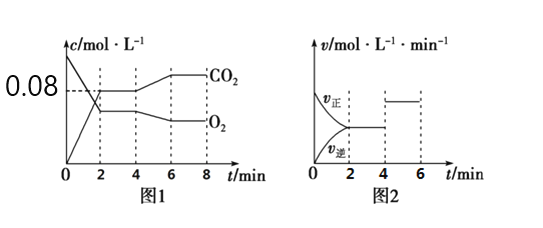
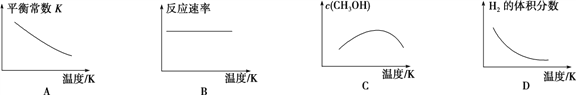
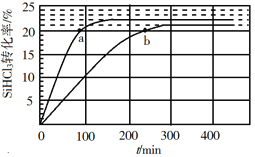
��Ŀ����
����Ŀ������������Ԫ��X��Y��Z��M��ԭ��������������Xԭ�Ӻ�������������������Ӳ�����2����X��Y�ĺ�������������֮��Ϊ2��3����������Z��Y2��ȼ�����ɵĻ��������ˮ����������ԭ��Ӧ��![]() �������Ϊ8���ӽṹ����ش��������⣺.
�������Ϊ8���ӽṹ����ش��������⣺.
��1��X������⻯��ķ���ʽΪ__________��
��2��Z������������ˮ��������_________����������������������
��3�����ȶ��ԣ�X������⻯���Y������⻯��_______������ǿ��������������
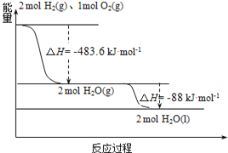
��4����������Z��Y2��ȼ�����ɻ����1mol�û�������ˮ��Ӧʱת�Ƶ�����Ϊ_______mol��
��5��Y��M��Ԫ��֮���γɵĻ����������ˮ������������_______�������ʽ����
���𰸡�CH4 �� �� 1 ClO2
��������
Xԭ�Ӻ�������������������Ӳ�����2����X��CԪ�أ�X��Y�ĺ�������������֮��Ϊ2��3��˵��Y������������Ϊ6��Y��OԪ�أ���������Z��Y2��ȼ�����ɵĻ��������ˮ����������ԭ��Ӧ��˵��Z��NaԪ�أ�![]() �������Ϊ8���ӽṹ��˵��M��ClԪ�ء�
�������Ϊ8���ӽṹ��˵��M��ClԪ�ء�
��1�����ݷ�����X��CԪ�أ�C������⻯��ķ���ʽΪCH4��
��2�����ݷ�����Z��NaԪ�أ�Na������������ˮ������NaOH����ǿ�
��3��X��CԪ�أ�Y��OԪ�أ��ǽ�����Խǿ���⻯��Խ�ȶ����ǽ�����C��O����CH4�����ȶ�������H2O��
��4��Na��O2��ȼ�����ɵ�Na2O2��ˮ��Ӧ2Na2O2+2H2O=4NaOH+O2 ����Na2O2��-1�۵�O�����绯����Ϊ0�ۺ�-2�ۣ���1mol Na2O2��ˮ��Ӧʱת�Ƶ�����Ϊ1mol��
��5���ȵ��������У�ClO2����Ϊˮ����������

 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д�����Ŀ���±���Ԫ�����ڱ���һ���֣����е�ÿ����ĸ����һ��Ԫ�أ������Ҫ��ش����⡣
�� ���� | ��A | 0 | |||||||
1 | a | ��A | ��A | ��A | ��A | ��A | ��A | ||
2 | b | c | d | ||||||
3 | e | f | g |
��1��Ԫ��g��Ԫ�����ڱ���λ��Ϊ____________________��
��2��b��g����Ԫ�ص�ԭ�Ӱ뾶��С��ϵ��b______g������>������<����.
��3����ԭ�Ӹ�����Ϊ1��1��1��a��b��c����Ԫ����ɵĹ��ۻ�����X�����γ�4�Թ��õ��Ӷԣ���X�ĽṹʽΪ______________��
��4��f�������������e������������Ӧ��ˮ��������Һ�з�Ӧ�����ӷ���ʽΪ_________________________��
��5��A��B��D��E������������Ԫ����ɵĻ��������֮���ת����ϵ��ͼ��ʾ�����ֲ�������ȥ����A��B��D����ɫ��Ӧ���ʻ�ɫ��ˮ��Һ��Ϊ���ԡ���ش�
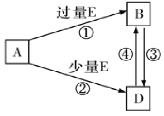
��E�ĵ���ʽΪ_______________��B�Ļ�ѧʽΪ____________________��
��A�еĻ�ѧ������Ϊ____________________
����Ȼ���д���B��D��H2O��һ�������ᾧ���ɵĹ��塣ȡһ�����ù�������ˮ���100mL��Һ�������Һ�н��������ӵ�Ũ��Ϊ0.5mo/L����ȡ��ͬ�����Ĺ���������������ٷ����仯��ʣ����������Ϊ___________g��
����Ŀ����ͨ����н�������ʵ�飺
ʵ�� ���� |
|
|
|
|
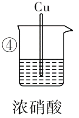
���� | Fe����������� ��ɫ���壬��Һ�� �Ϸ������ɫ | Fe�������� �Ա仯 | ����������ɫ���ݣ����Ⱥ�Cu���������ɫ���壬��Һ���Ϸ������ɫ | Cu��������� ��ɫ���� |
��ش��������⣺
��1��������������ɫ��Ϊ����ɫ������ɫ������__________________�������ʽ����
��2�����е�����˵��Fe������______��������ԭ����________________________��
��3���ԱȢ٢��е�����______����������������������˵��ϡ�����������ǿ��Ũ���ᡣ
��4���ԱȢۢ��е�����˵�������ԣ�ϡ����______������>������<����Ũ���ᡣ
��5�������ڼ���ʱ�Ļ�ѧ��Ӧ����ʽΪ________________________���˷�Ӧ��ϡ��������ֳ��������⣬�����ֳ�____________��