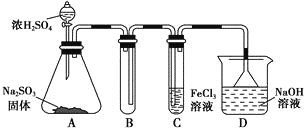
��Ŀ����
����Ŀ��ij��˾������һ���Լ״�Ϊԭ�ϣ���KOHΪ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���¡�����B�缫�ĵ缫����Ϊ̼����ͼ��һ���绯ѧ���̵�ʾ��ͼ������գ�
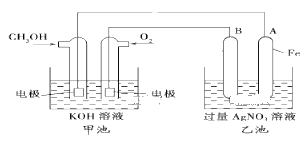
��1�����ʱ��ԭ��صĸ������Դ___���������ҳ��������ĵ缫��ӦΪ___��
��2���ŵ�ʱ�������ĵ缫��ӦʽΪ___��
��3���ڴ˹���������ȫ��Ӧ���ҳ���A������������648 g����׳�������������O2___L(��״����)��
��4�����ڳ��³�ѹ�£�1gCH3OHȼ������CO2��Һ̬H2Oʱ����22.68kJ����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ___��
���𰸡��� 4OH-+4e-=2H2O+O2�� CH3OH-6e-+8OH-=CO32-+6H2O 33.6 CH3OH��l��+![]() O2��g��=CO2��g��+2H2O��l�� ��H=-725.76kJ/mol
O2��g��=CO2��g��+2H2O��l�� ��H=-725.76kJ/mol
��������
��1���ŵ�ʱ�������ϼ״�ʧ���ӷ���������Ӧ��
��2�����ʱ��ԭ��ظ������Դ����������������ʧ���ӷ���������Ӧ��
��3���ҳ���B���������ӵõ��ӷ�����ԭ��Ӧ������ת�Ƶ�����ȼ��������������
��4��n��CH3OH��=![]() mol�����ȼ���ȵĸ�����д�Ȼ�ѧ����ʽ��
mol�����ȼ���ȵĸ�����д�Ȼ�ѧ����ʽ��
��1�����ʱ��ԭ��ظ������Դ��������������������������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��4OH--4e-�T2H2O+O2����
��2���ŵ�ʱ���״�ʧ���Ӻ����������ӷ�Ӧ����̼������Ӻ�ˮ�����Ե缫��ӦʽΪ��CH3OH-6e-+8OH-�TCO32-+6H2O��
��3���ҳ���B���������ӵõ��ӷ�����ԭ��Ӧ�����ҳ���B������������648g����׳�������������O2���= ��22.4L/mol=33.6L��
��22.4L/mol=33.6L��
��4��n��CH3OH��=![]() mol������CO2��Һ̬H2Oʱ����22.68kJ����1molCH3OHȼ�շų�������Ϊ1��22.68kJ��32=725.76kJ����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪCH3OH��l��+
mol������CO2��Һ̬H2Oʱ����22.68kJ����1molCH3OHȼ�շų�������Ϊ1��22.68kJ��32=725.76kJ����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪCH3OH��l��+![]() O2��g��=CO2��g��+2H2O��l�� ��H=-725.76kJ/mol��
O2��g��=CO2��g��+2H2O��l�� ��H=-725.76kJ/mol��

����Ŀ���������и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת������
a | b | c | |
A | Al2O3 | AlCl3 | Al(OH)3 |
B | NH3 | NO | NO2 |
C | Si | SiO2 | H2SiO3 |
D | Fe | FeCl2 | FeCl3 |
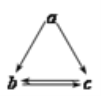
A.AB.BC.CD.D