��Ŀ����
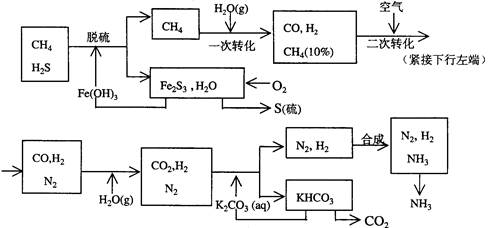
��14�֣���ҵ��������Ĺ�������ʾ��ͼ���£�

���������գ�
��1������ˮ���������A��B�����ʣ�������A��Դ��ʯ��Ҥ������ʵ�����ᴿ���ε�ʵ���������Ϊ��ȡ�����ܽ⡢������������������������ȴ�ᾧ��������������ɡ�
��2����ҵ��������������У�̼�ữʱ����������������������������������������
̼�ữʱû������̼���ƾ��壬��ԭ���ǡ�����������������������������
��3��̼�ữ����ˣ���ҺD����Ҫ�ijɷ�����������������������������������д��ѧʽ����������һ�ɷֵ������ӵľ��巽���ǣ�����������������������������
��4����������а���ѭ��ʹ�õģ�Ϊ�ˣ���ҺD����ʯ�������������ʯ��ˮ���������ķ�Ӧ�����ӷ���ʽΪ����������������������������������������������������
��5����Ʒ�����к���̼�����ơ�ȡa�˲�Ʒ�ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�������������ּ��Ȳ��ڸ���������ȴ��b�˹��塣 ������̼�����Ƶ����������ɱ�ʾΪ�� ��

���������գ�
��1������ˮ���������A��B�����ʣ�������A��Դ��ʯ��Ҥ������ʵ�����ᴿ���ε�ʵ���������Ϊ��ȡ�����ܽ⡢������������������������ȴ�ᾧ��������������ɡ�
��2����ҵ��������������У�̼�ữʱ����������������������������������������
̼�ữʱû������̼���ƾ��壬��ԭ���ǡ�����������������������������
��3��̼�ữ����ˣ���ҺD����Ҫ�ijɷ�����������������������������������д��ѧʽ����������һ�ɷֵ������ӵľ��巽���ǣ�����������������������������
��4����������а���ѭ��ʹ�õģ�Ϊ�ˣ���ҺD����ʯ�������������ʯ��ˮ���������ķ�Ӧ�����ӷ���ʽΪ����������������������������������������������������
��5����Ʒ�����к���̼�����ơ�ȡa�˲�Ʒ�ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�������������ּ��Ȳ��ڸ���������ȴ��b�˹��塣 ������̼�����Ƶ����������ɱ�ʾΪ�� ��
��14�֣�
��1�� ���� ���� (ÿ��1��)
��2���о�������������ֻ��ǣ� ��2�֣�̼�����ܽ�Ƚϴ�2�֣�
��3��NH4Cl��1�֣�ȡ�����������ữ���������������а�ɫ���������������ӡ���2�֣�
��4��NH4++ OH-= NH3��+ H2O����2NH4++ Ca(OH��2= 2NH3��+ H2O+Ca2+Ҳ����)��2�֣�
��5�� [168��a-b��/62a] 100��(Ҳ�����ǻ�����ʽ��)��3�֣�
��1�� ���� ���� (ÿ��1��)
��2���о�������������ֻ��ǣ� ��2�֣�̼�����ܽ�Ƚϴ�2�֣�
��3��NH4Cl��1�֣�ȡ�����������ữ���������������а�ɫ���������������ӡ���2�֣�
��4��NH4++ OH-= NH3��+ H2O����2NH4++ Ca(OH��2= 2NH3��+ H2O+Ca2+Ҳ����)��2�֣�
��5�� [168��a-b��/62a] 100��(Ҳ�����ǻ�����ʽ��)��3�֣�
��1��ʵ�����ᴿ���ε�ʵ���������Ϊ��ȡ�����ܽ⡢���������ˡ���������ȴ�ᾧ�����ˡ���ɡ�
��2����ҵ��������������У�̼�ữʱ�����������ǣ��о��� ������̼�ữʱû������̼���ƾ��壬��ԭ����̼���Ƶ��ܽ�Ƚϴ�
������̼�ữʱû������̼���ƾ��壬��ԭ����̼���Ƶ��ܽ�Ƚϴ�
��3��̼�ữ����ˣ���ҺD����Ҫ�ijɷ��ǣ�NH4Cl��������һ�ɷֵ������ӵľ��巽���ǣ�ȡ�����������ữ���������������а�ɫ���������������ӡ�
��4����ʯ��ˮ���������ķ�Ӧ�����ӷ���ʽΪ��NH4++ OH-= NH3��+ H2O����2NH4++ Ca(OH��2= 2NH3��+ H2O+Ca2+��
��5��������̼�����Ƶ����������ɱ�ʾΪ��[168��a-b��/62a] ��100��
��2����ҵ��������������У�̼�ữʱ�����������ǣ��о���
 ������̼�ữʱû������̼���ƾ��壬��ԭ����̼���Ƶ��ܽ�Ƚϴ�
������̼�ữʱû������̼���ƾ��壬��ԭ����̼���Ƶ��ܽ�Ƚϴ���3��̼�ữ����ˣ���ҺD����Ҫ�ijɷ��ǣ�NH4Cl��������һ�ɷֵ������ӵľ��巽���ǣ�ȡ�����������ữ���������������а�ɫ���������������ӡ�
��4����ʯ��ˮ���������ķ�Ӧ�����ӷ���ʽΪ��NH4++ OH-= NH3��+ H2O����2NH4++ Ca(OH��2= 2NH3��+ H2O+Ca2+��
��5��������̼�����Ƶ����������ɱ�ʾΪ��[168��a-b��/62a] ��100��

��ϰ��ϵ�д�
 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
�����Ŀ
 -﮻Կ�(LiAlSi2O6��������þ����)��Ӧ������Li2SO4��MgSO4�ȣ������Ʊ�����ﮡ�
-﮻Կ�(LiAlSi2O6��������þ����)��Ӧ������Li2SO4��MgSO4�ȣ������Ʊ�����ﮡ� -﮻Կ�������������Ʊ�����﮵Ĺ�ҵ��������ͼ��
-﮻Կ�������������Ʊ�����﮵Ĺ�ҵ��������ͼ��

 2MgCl2��Ti��Ar������������__________________
2MgCl2��Ti��Ar������������__________________