��Ŀ����
�����������漰�϶�Ļ�ѧԭ�������������������̣���ش��������⣺
��1����ҵ���������������������Ҫ�豸��__________��__________��__________��
��2����ҵ���Ʊ����ᣬ������__________�����ţ�Ϊ���ռ����Ը�Ч������������
a.����ˮ b.����ˮ c.ϡ���� d.98.3%����
��3�������Ƽ�У��ö�����̼�Ͱ����뱥��ʳ��ˮ��Ӧ��̼�����ƣ�Ӧ����ʳ��ˮ��ͨ��_____��
��4��ʯ���ѻ���Ϊ���ѻ������ѻ���_______����Ŀ��Ϊ__________________��
��5��Ϊ���͵��Һ�۵㣬��ҵ��������ʱ��Ҫ������ۼ�Ϊ____________________��
��1����ҵ���������������������Ҫ�豸��__________��__________��__________��
��2����ҵ���Ʊ����ᣬ������__________�����ţ�Ϊ���ռ����Ը�Ч������������
a.����ˮ b.����ˮ c.ϡ���� d.98.3%����
��3�������Ƽ�У��ö�����̼�Ͱ����뱥��ʳ��ˮ��Ӧ��̼�����ƣ�Ӧ����ʳ��ˮ��ͨ��_____��
��4��ʯ���ѻ���Ϊ���ѻ������ѻ���_______����Ŀ��Ϊ__________________��
��5��Ϊ���͵��Һ�۵㣬��ҵ��������ʱ��Ҫ������ۼ�Ϊ____________________��
��ÿ��1�֣�8�֣���1������¯���Ӵ��ҡ������� ��2��d ��3������
��4�������ѻ����������Һ��ȼ�ϣ����ͣ�ú�ͣ����͵ȣ��IJ������ر���������͵IJ���
��5��Na3AlF6�����ʯ��������������
��4�������ѻ����������Һ��ȼ�ϣ����ͣ�ú�ͣ����͵ȣ��IJ������ر���������͵IJ���
��5��Na3AlF6�����ʯ��������������
�����������1����ҵ�����������Ҫ��Ӧ���£��ڷ���¯�з�Ӧ��4FeS2+11O2
 2Fe2O3+8SO2���ڽӴ����ڷ�Ӧ��2SO2+O2
2Fe2O3+8SO2���ڽӴ����ڷ�Ӧ��2SO2+O2 2SO3�����������ڷ�Ӧ��SO3+H2O��H2SO4�����Թ�ҵ���������������������Ҫ�豸�з���¯���Ӵ��ҡ���������
2SO3�����������ڷ�Ӧ��SO3+H2O��H2SO4�����Թ�ҵ���������������������Ҫ�豸�з���¯���Ӵ��ҡ�����������2������������ˮ����SO3���ײ����������谭SO3�����գ���������������98.3%��Ũ��������SO3��
��3����Ϊ������ˮ��Һ�ʼ��ԣ��������ն�����̼�������ư���ˮ�Ĺ�����Ҫ��ͨ�백������ͨ�������̼��
��4��ʯ���ѻ���Ϊ���ѻ������ѻ��ͼ����ѻ���ʯ���ѻ���Ŀ����Ϊ���������Һ��ȼ�ϣ����ͣ�ú�ͣ����͵ȣ��IJ������ر���������͵IJ�����
��5�����������۵�ܸߣ�����Ϊ���͵��Һ�۵㣬��ҵ��������ʱ��Ҫ������ۼ�Ϊ����ʯ����ѧʽΪ��Na3AlF6����

��ϰ��ϵ�д�
�����Ŀ
 2NH3��g��������ӦΪ���ȷ�Ӧ��������˵������ȷ���ǣ���������
2NH3��g��������ӦΪ���ȷ�Ӧ��������˵������ȷ���ǣ��������� ���������������£���l000ag CaCO3��60ag SiO2��ϣ������ɵ�CO2�ڱ�״���µ����Ϊ ���ú�a�Ĵ���ʽ��ʾ����
���������������£���l000ag CaCO3��60ag SiO2��ϣ������ɵ�CO2�ڱ�״���µ����Ϊ ���ú�a�Ĵ���ʽ��ʾ����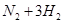


 ��
��


 2NO(g) ��H
2NO(g) ��H ��������ͬ���������ʱ����Һ�����ԣ���������pH__________
��������ͬ���������ʱ����Һ�����ԣ���������pH__________ ������ڡ�����С�ڡ����ڡ�����
������ڡ�����С�ڡ����ڡ�����