��Ŀ����
[��ѧһѡ��2:��ѧ�뼼��](15�֣���֪��
�ٹ�ҵ���������� -﮻Կ�(LiAlSi2O6��������þ����)��Ӧ������Li2SO4��MgSO4�ȣ������Ʊ�����ﮡ�
-﮻Կ�(LiAlSi2O6��������þ����)��Ӧ������Li2SO4��MgSO4�ȣ������Ʊ�����ﮡ�
�����������һ���¶��¿�ת��Ϊ����ﮡ��� -﮻Կ�������������Ʊ�����﮵Ĺ�ҵ��������ͼ��
-﮻Կ�������������Ʊ�����﮵Ĺ�ҵ��������ͼ��

��ش�
(1)����Y����Ҫ�ɷֵĻ�ѧʽ��____________��
(2)������ʹ���˲�ͬŨ�ȵ�Na2CO3��Һ���������ܽ�ȴ�С�ĽǶȽ�����ʹ�õ�ԭ��____________
(3 )����1�漰��ʵ�鷽����__________________��
(4 )д�������ۻ�ԭ����ȡ����﮵Ļ�ѧ��Ӧ����ʽ��________________________
(5) LiCl��Һ�������ɺ����ù���������״̬�µ���Ʊ�ﮡ����ʱ�����������л�����������������������ԭ����__________________
(6) Ŀǰ���ͨ��ʹ�ý���﮵��ŵ���__________________
�ٹ�ҵ����������
 -﮻Կ�(LiAlSi2O6��������þ����)��Ӧ������Li2SO4��MgSO4�ȣ������Ʊ�����ﮡ�
-﮻Կ�(LiAlSi2O6��������þ����)��Ӧ������Li2SO4��MgSO4�ȣ������Ʊ�����ﮡ������������һ���¶��¿�ת��Ϊ����ﮡ���
 -﮻Կ�������������Ʊ�����﮵Ĺ�ҵ��������ͼ��
-﮻Կ�������������Ʊ�����﮵Ĺ�ҵ��������ͼ��
��ش�
(1)����Y����Ҫ�ɷֵĻ�ѧʽ��____________��
(2)������ʹ���˲�ͬŨ�ȵ�Na2CO3��Һ���������ܽ�ȴ�С�ĽǶȽ�����ʹ�õ�ԭ��____________
(3 )����1�漰��ʵ�鷽����__________________��
(4 )д�������ۻ�ԭ����ȡ����﮵Ļ�ѧ��Ӧ����ʽ��________________________
(5) LiCl��Һ�������ɺ����ù���������״̬�µ���Ʊ�ﮡ����ʱ�����������л�����������������������ԭ����__________________
(6) Ŀǰ���ͨ��ʹ�ý���﮵��ŵ���__________________
(1) Li2CO3��2�֣�
(2) CaCO3���ܽ��С��Li2CO3����ϡNa2CO3��Һ���ܳ�ȥ��Һ�е�Ca2+���ֲ�����Li2CO3��������2�֣��ӱ���Na2CO3��Һ��Ŀ����ʹLi +ת������Li2CO3��������2�֣�
(3) ���� ϴ�ӣ�2�֣� (4)2Al+3Li2O 6Li + Al2O3 ��2�֣�
6Li + Al2O3 ��2�֣�
��5��LiCl��Һ�ڼ������ɹ������в���LiOH���ɣ�LiOH���ȷֽ�ΪLi2O�����ڵ�Li2O���������������3�֣�
��6��﮵������ᣬ����λ�ߡ��������ߣ�﮵��Ϊ�ɳ����ɫ������ء���2�֣�����������Ҳ���֣�
(2) CaCO3���ܽ��С��Li2CO3����ϡNa2CO3��Һ���ܳ�ȥ��Һ�е�Ca2+���ֲ�����Li2CO3��������2�֣��ӱ���Na2CO3��Һ��Ŀ����ʹLi +ת������Li2CO3��������2�֣�
(3) ���� ϴ�ӣ�2�֣� (4)2Al+3Li2O
 6Li + Al2O3 ��2�֣�
6Li + Al2O3 ��2�֣���5��LiCl��Һ�ڼ������ɹ������в���LiOH���ɣ�LiOH���ȷֽ�ΪLi2O�����ڵ�Li2O���������������3�֣�
��6��﮵������ᣬ����λ�ߡ��������ߣ�﮵��Ϊ�ɳ����ɫ������ء���2�֣�����������Ҳ���֣�
�����Թ�ҵ���Ϊ���忼���˳����ܽ�ƽ�⣬ˮ�⡢ԭ��غ͵������ԭ��
��1������﮺�̼���Ʊ�����Һ��Ӧ������̼��﮳����������ƣ�����Y���յIJ���������﮿�֪�� Y��̼��ﮡ�
��2��X��������þ��̼��Ƴ���������CaCO3���ܽ��С��Li2CO3����ϡNa2CO3��Һ���ܳ�ȥ��Һ�е�Ca2+���ֲ�����Li2CO3�������������ӱ���Na2CO3��Һ��Ŀ����ʹLi +ת������Li2CO3������
��3������Һ�з��������ķ���Ӧ�ǹ��ˣ����˺������ϴ�ӡ�
��4���ڼ��ȵ������£������Է������ȷ�Ӧ������ʽΪ2Al+3Li2O 6Li + Al2O3��
6Li + Al2O3��
��5������O2��ԭ�����ӷ�Ӧ����������Ѱ�ң����ڼ�������LiCl��Һ�ɻ�ù��壬��������LiCl������ˮ���LiOH��LiOH���ȷֽ������Li2O���ʱ�����O2��
��6��﮵������ᣬ����λ�ߡ��������ߣ���﮵��Ϊ�ɳ����ɫ������ء�
��1������﮺�̼���Ʊ�����Һ��Ӧ������̼��﮳����������ƣ�����Y���յIJ���������﮿�֪�� Y��̼��ﮡ�
��2��X��������þ��̼��Ƴ���������CaCO3���ܽ��С��Li2CO3����ϡNa2CO3��Һ���ܳ�ȥ��Һ�е�Ca2+���ֲ�����Li2CO3�������������ӱ���Na2CO3��Һ��Ŀ����ʹLi +ת������Li2CO3������
��3������Һ�з��������ķ���Ӧ�ǹ��ˣ����˺������ϴ�ӡ�
��4���ڼ��ȵ������£������Է������ȷ�Ӧ������ʽΪ2Al+3Li2O
 6Li + Al2O3��
6Li + Al2O3����5������O2��ԭ�����ӷ�Ӧ����������Ѱ�ң����ڼ�������LiCl��Һ�ɻ�ù��壬��������LiCl������ˮ���LiOH��LiOH���ȷֽ������Li2O���ʱ�����O2��
��6��﮵������ᣬ����λ�ߡ��������ߣ���﮵��Ϊ�ɳ����ɫ������ء�

��ϰ��ϵ�д�
 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д� Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д�
�����Ŀ
 2NH3��g��������ӦΪ���ȷ�Ӧ��������˵������ȷ���ǣ���������
2NH3��g��������ӦΪ���ȷ�Ӧ��������˵������ȷ���ǣ���������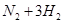


 ��
��


 2NO(g) ��H
2NO(g) ��H ��������ͬ���������ʱ����Һ�����ԣ���������pH__________
��������ͬ���������ʱ����Һ�����ԣ���������pH__________ ������ڡ�����С�ڡ����ڡ�����
������ڡ�����С�ڡ����ڡ�����

