��Ŀ����
����Ŀ��A��B��C��DΪ���ֶ�����Ԫ�أ����ǵ�ԭ������������������A��C��B��D�ֱ���ͬ����Ԫ�أ���֪B��D��Ԫ�ؼ���γ�DB2��DB3���ֻ����������Է����������16����֪A��C��Ԫ��ԭ������֮����B��D��Ԫ��ԭ������֮�͵�![]() ����ش��������⣺
����ش��������⣺
(1)д����A��B��C����Ԫ���γɵĻ�����ĵ���ʽ��__________________________���侧����������ѧ����������________________________________________________________��
(2)A2B�ķе��A2D�ķе�(����������������)________����ԭ����_________________��
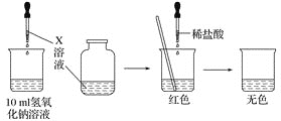
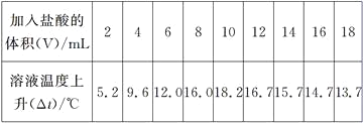
(3)��A��B��C��D����Ԫ���γɵ�����X�������ᷴӦ�ܹ����ɾ��д̼�����ζ�����壬д��X�����ᷴӦ�����ӷ���ʽ��___________________________________________________��
���𰸡�![]() ���Ӽ������ۼ� �� H2O����֮�����γ������ʹˮ�ķе��H2S�ĸ� HSO3-��H��
���Ӽ������ۼ� �� H2O����֮�����γ������ʹˮ�ķе��H2S�ĸ� HSO3-��H��![]() SO2����H2O
SO2����H2O
��������
��֪B��D��Ԫ�ؼ���γ�DB2��DB3���ֻ����������Է����������16������Ƴ�B�����ԭ������Ϊ16������BΪOԪ�أ�����ΪB��Dͬ���壬��D��ԭ����������B������DΪSԪ�أ�B��D��ԭ������֮�͵�1/2��12������ΪA��Cͬ���壬����AΪHԪ�أ�CΪNaԪ�أ��ݴ˴��⡣
�ɷ�����֪��AΪHԪ�أ�BΪOԪ�أ�CΪNaԪ�أ�DΪSԪ�ء�
��1��A��B��C����Ԫ���γɵĻ�����ΪNaOH��NaOH�ĵ���ʽΪ��![]() �����������������Ӻ����������������Ӽ����ϣ�������Թ��ۼ�����γ����������ӣ��ʴ�Ϊ��
�����������������Ӻ����������������Ӽ����ϣ�������Թ��ۼ�����γ����������ӣ��ʴ�Ϊ��![]() �����Ӽ������ۼ���
�����Ӽ������ۼ���
��2��H2O��H2S�����⻯�����嶼���ڷ��Ӿ��壬���Ӿ��������ʵķе��������ԭ�����������ȣ���ˮ���Ӽ京�������������Ӽ䲻����������Զ��ߵķе�ϸߵ���H2O���ʴ�Ϊ���ߣ�H2O����֮�����γ������ʹˮ�ķе��H2S�ĸߡ�
��3����A��B��C��D����Ԫ���γɵ����ʿ�����NaHSO4��NaHSO3������X�����ᷴӦ�ܹ����ɾ��д̼�����ζ�����壬��XΪNaHSO3��NaHSO3��HCl��Ӧ�����ӷ���ʽΪ��HSO3-��H��=SO2����H2O���ʴ�Ϊ��HSO3-��H��=SO2����H2O��