��Ŀ����
����Ŀ�������£���0.100 mol��L��1 NaOH��Һ�ζ�10 mL 0.100 mol��L��1 H3PO4��Һ��������ͼ��ʾ������˵����ȷ����
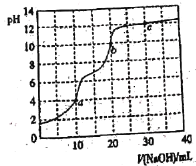
A.�ζ��յ�a��ѡ���̪��ָʾ��
B.c����Һ��c(Na��)>3c(PO43��)��2c(HPO42��)��c(H2PO4��)
C.b����Һ��c(HPO42��)>c(PO43��)>c(H2PO4��)
D.a��b��c������ˮ�ĵ���̶���С����c
���𰸡�B
��������
NaOH�ζ�������������η�����Ӧ��NaOH+H3PO4=NaH2PO4+H2O��NaOH+ NaH2PO4=Na2HPO4+H2O��NaOH+Na2HPO4=Na3PO4+H2O�����ζ�Ӧ����10mLNaOH��20mLNaOH��30mLNaOH��
A����ͼ��֪a���pH=4����̪�ı�ɫΪ8.2~10�������÷�̪��ָʾ�������ʣ���A����
B��c�����30mLNaOH����Һ�е�����ΪNa3PO4�����������غ��֪c(Na��)=3c(PO43��)��3c(HPO42��)��3c(H2PO4��)+3c(H3PO4)������c(Na��)>3c(PO43��)��2c(HPO42��)��c(H2PO4��)����B��ȷ��
C��b�����20mLNaOH����Һ�е�����ΪNa2HPO4����ʱ��Һ�Լ��ԣ�˵��HPO42-��ˮ��̶ȴ��������̶ȣ�����c(H2PO4��)> c(PO43��)����C����
D������ĵ�������ˮ�ĵ��룬�����ˮ��ٽ�ˮ�ĵ��룬��c�㴦����ֻ��Na3PO4����ʱֻ���������ˮ��ٽ����룬ˮ�ĵ���̶����D����
�ʴ�ΪB��

����Ŀ���������ִ���ѧ��ҵ��ռ�м�����Ҫ�ĵ�λ����Ҫ�漰����Ԫ�ؼ��仯����衢��������ȡ��й���ѧ�Ҵ����Եع����˹軯�ᄃ������ĵ������Ĵ������ɹ���ʵ���˼���������������ѡ����һ����Ч������ϩ�������������Ȼ�ѧƷ��
(1)�衢̼λ��ͬһ���壬�á�>����<��������գ�
���� | ԭ�Ӱ뾶 | ��һ������ | �۵� | ���� |
��Ŀ | ��Si__________C | ��C_________Si | ��CO2______SiO2 | ��H-Si_______H-C |
(2)CN������Fe3���γ�������CN����Ϊ�ȵ�����ķ�����_____________(��дһ��)��1mol[Fe(CN)6]3���к�____________mol ������
(3)��֪����Ӧ2CH4![]() CH2��CH2��2H2��̼ԭ�ӵ��ӻ�����ת������Ϊ___________��
CH2��CH2��2H2��̼ԭ�ӵ��ӻ�����ת������Ϊ___________��
(4)���ķ���ϩ��һ�����壬������һ����ƽ�����������ϸ�����λ����Ķ��ؾ��壬��ͨ��___________�������־��塢����ͷǾ��塣
(5)��̬Fԭ�ӵļ۲�����Ų�ͼΪ___________��
(6)[H2F]��[SbF6]��(������)��һ�ֳ�ǿ�ᣬ����[H2F]���������ӵĿռ乹��Ϊ____________��
(7)CuCl���۵�Ϊ426�����ۻ�ʱ���������磻CuF���۵�Ϊ908�����ܶ�Ϊ7.1g��cm��3��
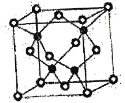
��CuF���۵��CuCl�ĸߣ�ԭ����____________________________________________��
����֪NAΪ�����ӵ�������ֵ��CuF�ľ����ṹ��ͼ��ʾ����CuF�ľ�������a��__________nm(�г�����ʽ)��
����Ŀ����ͼ�����ʼ䷢����ѧ��Ӧ����ɫ�仯���±�ѡ�����ʶ�Ӧ��ȷ����

ѡ�� | M | N | P | Q |
A | NH3��H2O | Ba(OH)2 | ͭ | KSCN |
B | Na | BaCl2 | FeO | KSCN |
C | NaOH | Ba(NO3)2 | � | KSCN |
D | Na2O2 | MgCl2 | Fe | KSCN |
A.AB.BC.CD.D