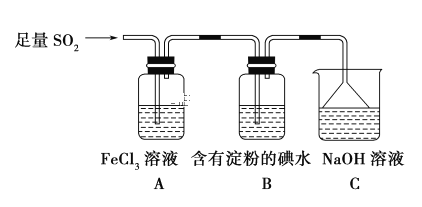
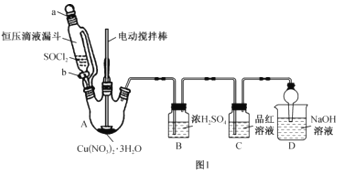
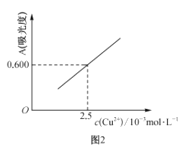
��Ŀ����
����Ŀ����NAΪ�����ӵ�����ֵ������˵����ȷ����
A.�����£�1 L pH=9��CH3COONa��Һ�У����������ˮ������Ϊ1��10-9NA
B.�����£�10 mL 5.6 mol/L FeC13��Һ�ε�100 mL��ˮ�У����ɽ�����Ϊ0.056NA
C.��Na2O2ͨ��������ˮ������������������bg���÷�Ӧת�Ƶ�����Ϊ![]()
D.6.8 g KHSO4�����к��е�������Ϊ0.15 NA
���𰸡�C
��������
A. �����£�pH=9��CH3COONa��Һ��ˮ�����������������Ũ����![]() ��1 L pH=9��CH3COONa��Һ�У����������ˮ������Ϊ1��10-5NA����A����
��1 L pH=9��CH3COONa��Һ�У����������ˮ������Ϊ1��10-5NA����A����
B. ���������������������������ľۼ��壬�����£�10 mL 5.6 mol/L FeC13��Һ�ε�100 mL��ˮ�У����ɽ�����С��0.056NA����B����
C. ![]() ���ɷ���ʽ��֪����������������������������������4gת��2mol���ӣ���Na2O2ͨ��������ˮ������������������bg���÷�Ӧת�Ƶ�����Ϊ
���ɷ���ʽ��֪����������������������������������4gת��2mol���ӣ���Na2O2ͨ��������ˮ������������������bg���÷�Ӧת�Ƶ�����Ϊ![]() ����C��ȷ��
����C��ȷ��
D. KHSO4���庬��K+��HSO4-�� 6.8 g KHSO4�����к��е�������Ϊ![]() 0.1 NA����D����
0.1 NA����D����
ѡC��

����Ŀ����1������___________________��
��2������Ũ�ȣ�_________________________
���¶ȣ�________________________________________
������
��3����ʽ����Һ������ԣ�ǿ����ʽ����__________��ԭ����_____________________________����Ԫ������ʽ����Һ�������ȡ����_____________________________________������NaHCO3��Һ�Լ��ԣ�ԭ����_________________��_______________________________�����ӷ���ʽ������˵������
NaHSO3��Һ�����Ե�ԭ����________________��_____________________�����ӷ���ʽ������˵������
��4��������������������ʾ�������CH3COONa��Һ�ı�������������д�仯�����CH3COO-��H2O![]() CH3COOH��OH��_______________
CH3COOH��OH��_______________
�ı����� | ƽ���ƶ� | c(CH3COO-) | c(OH-) | ˮ�� |
����CH3COONa | ||||
ͨ��HCl | ||||
���� | ||||
��ˮ | ||||
��NaOH | ||||
��HAc | ||||
��NH4Cl |