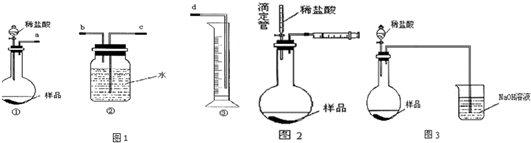
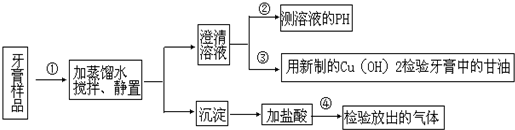
��Ŀ����
10��������Ԫ��X��Y��Z�����ڱ���λ�ù�ϵ��ͼ��| X | ||
| Y | ||
| Z |
��1��xԪ�صĵ��ʷ���ʽ��He��������ԭ�Ӿ��壮
��2����Ȼ���д���һ�ֽ�������Y����Ԫ�ص���Ȼ��������ʽΪ
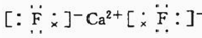 ���������Ӿ��壮
���������Ӿ��壮��3��Z���⻯���Y���⻯��е�ߵ�˳���ǣ�HF��H2S�����ɣ�HF����֮����˴��ڷ��Ӽ��������������������
��4��X��Y��Z����Ԫ���У��縺�������ǣ�F��
���� �ɶ����ڼ�Ԫ�ص�λ�ÿ�֪��XΪHe����YΪF��ZΪS��
��1��ϡ������Ϊ��ԭ�ӷ��ӣ��������ʽΪHe�����ڷ��Ӿ��壻
��2��YΪFԪ�أ������γɵĻ�����Ϊ�����ƣ��������ӻ����
��3��Z���⻯���Y���⻯��ֱ�Ϊ����ͷ����⣬�������д��������������ķе�������⣻
��4���ǽ�����Խǿ���縺��Խǿ��F�ǵ縺����ǿ��Ԫ�أ�
��� �⣺�ɶ����ڼ�Ԫ�ص�λ�ÿ�֪��XΪHe����YΪF��ZΪS��
��1��XΪHe������ϡ������Ԫ�أ������ʽΪ��He��He����Ϊ���Ӿ��壬
�ʴ�Ϊ��He�����ӣ�
��2����Ȼ���д���һ�ֽ�������Y����Ԫ�ص���Ȼ���YΪFԪ�أ������γɵĻ�����Ϊ�����ƣ��������������ӻ���������ʽΪ��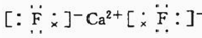 ��
��
�ʴ�Ϊ��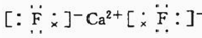 �� ���ӣ�
�� ���ӣ�
��3��Z���⻯���Y���⻯��ֱ�Ϊ����ͷ�����ֱ�Ϊ����ͷ����⣬���� HF����֮����˴��ڷ��Ӽ�����������������������Է�����ķе�ϸߣ�����HF��H2S��
�ʴ�Ϊ��HF��H2S��HF����֮����˴��ڷ��Ӽ��������������������
��4��He��S��FԪ���У��ǽ�������ȷ��ΪF����縺����ǿ��ΪF��
�ʴ�Ϊ��F��
���� ���⿼��Ԫ�������ɺ�Ԫ�����ڱ�����Ŀ�Ѷ��еȣ�ע����ϤԪ�������ڱ��е�λ�ã���ȷԪ�����������Ա仯��ԭ��Ԫ�����ʵ������Ա仯�ǽ����Ĺؼ���

| ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� |
| A | ����Һ��������������ʱ���ò���������Ħ�����ڣ� | ��ʹ�������� |
| B | ��һ���ȥ����Ĥ����Ƭ����Ũ�����У�һ��ʱ���ȡ��ϴ�����ٷ���һ��Ũ�ȵ�CuSO4��Һ�У� | ��֤Al��Ũ�����еĶۻ� |
| C | ��ʳ�����Ậ���ⶨʵ���У���25mL��Һ����ȡ����ʳ��25mL������250mL����ƿ�У���ˮϡ�����̶��ߣ�ҡ�ȵô���ʳ����Һ | ��Ҫ�Ƿ�ֹʳ�Ļӷ�������ʵ���� |
| D | ������̼�����ھƾ���ƻ���������2��3���Ӻ�����Ͷ�뵽��ˮ�У���������3��5�Σ� | ����ʹ̼�������ôֲڶ�ף��������ո�������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��������Ͷ��ˮ�У�Na+2H2O�TNa++OH-+H2�� | |
| B�� | ����CO2ͨ��Ca��ClO��2��Һ�У�ClO-+CO2+H2O�THCO3-+HCIO | |
| C�� | NH4HCO3��Һ�������NaOH��Һ��Ӧ��NH4++OH-�TNH3��+H2O | |
| D�� | ͭƬ����ϡ�����У�Cu+4H++2NO3-�TCu2++2NO2��+2H2O |
| A�� | ú�ĸ��� | B�� | �ѽ� | ||
| C�� | ��ú�����еõ������ױ������ױ��� | D�� | �Ŵ�ֲ����ʳ�ú |
| A�� | 25��ʱ��PH=13��1.0L Ba��OH��2��Һ�к��е�OH-��ĿΪ0.2NA | |
| B�� | �����£�0.100 mol•L-1̼������Һ�У�CO32-��ĿС��0.2NA | |
| C�� | �����£�21.0g��ϩ�Ͷ�ϩ�Ļ�������к��е�̼ԭ����ĿΪ1.5NA | |
| D�� | ��״���£�22.4L �״��к��е���ԭ����Ϊ1.0NA |
| A�� | ������ʴʱ���ܷ�����������Ӧ��2H2O+O2+4e-�T4OH- | |
| B�� | ����ˮ������ӷ���ʽ��Al3++3 H2O?Al��OH��3+3H+ | |
| C�� | ��������������Һ��̼��������Һ��ϣ�OH-+HCO3-�TH2O+CO32- | |
| D�� | ��ʾ����ȼ�յ��Ȼ�ѧ����ʽ2H2��g��+O2��g���T2H2O��l������H=-571.6kJ•mol-1 |