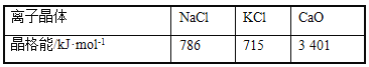
ЬтФПФкШн
ЁОЬтФПЁПОЙ§ЕТЙњЛЏбЇМвЙўВЎЁЂВЈЪЉЕШЕФВЛаИХЌСІЃЌГЩЙІЕиПЊЗЂСЫКЯГЩАБЕФЩњВњЙЄвеЁЃШчНё ЪРНчИїЙњПЦбЇМвЮЊЬсИпАБЕФВњСПЃЌНЕЕЭФмКФзізХИїжжгавцЕФЬНЫїЁЃЪдЛиД№ЯТСаЮЪЬтЃК
(1)вбжЊNH3(l)NH3(g) ЁїH1ЃЛN2(g)+3H2(g)2NH3(l) ЁїH2 ЁЃдђЗДгІN2(g)+3H2(g)2NH3(g) ЕФЁїH=_______(гУКЌ ЁїH1ЁЂЁїH2ЕФДњЪ§ЪНБэЪО)
(2)ЂйдквЛЖЈЬѕМўЯТЃЌЗжБ№НЋ 1mol N2КЭ3mol H2жУгкКубЙШнЦїЂёКЭКуШнШнЦїЂђжа СНШнЦїЦ№ЪМШнЛ§ЯрЭЌГфЗжЗДгІЃЌЖўепОљДяЕНЦНКтзДЬЌЃЌдђСНШнЦїжаNH3 ЕФЬхЛ§ЗжЪ§ЪЧЂё_______Ђђ(ЬюЁАЃОЁБЁЂЁАЃМЁБЛђЁА=ЁБ ЁЃ)
ЂкЩЯЪіШнЛ§КуЖЈЕФУмБеШнЦїжаЃЌДяЛЏбЇЦНКтзДЬЌЪБЃЌ ШєNH3 ЕФЬхЛ§ЗжЪ§ЮЊ10% ЃЌШєБЃГжЦфЫћЬѕ МўВЛБфЃЌЦ№ЪМЪБИФЮЊГфШы2 mol N2КЭ2molH2ЃЌДяаТЦНКтКѓЃЌNH3ЕФЬхЛ§ЗжЪ§ЮЊ_______10%Ью(ЁАЃОЁБЁЂЁАЃМЁБЛђЁА=ЁБ)ЁЃ
(3)ЙўВЎвђжЄЪЕ N2ЁЂH2 дкЙЬЬхДпЛЏМС(Fe)БэУцЮќИНКЭНтЮќвдКЯГЩАБЕФЙ§ГЬЖјЛёХЕБДЖћНБЁЃШєгУ![]() ЗжБ№БэЪО N2ЁЂH2ЁЂNH3 КЭЙЬЬхДпЛЏМСЃЌдђдкЙЬЬхДпЛЏМСБэУцКЯГЩАБЕФЙ§ГЬПЩгУШчЭМБэЪОЃК
ЗжБ№БэЪО N2ЁЂH2ЁЂNH3 КЭЙЬЬхДпЛЏМСЃЌдђдкЙЬЬхДпЛЏМСБэУцКЯГЩАБЕФЙ§ГЬПЩгУШчЭМБэЪОЃК
![]()
ЂйЮќИНКѓЃЌФмСПзДЬЌзюЕЭЕФЪЧ___________ЬюзжФИађКХ)ЁЃ
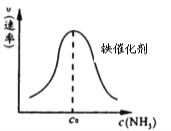
ЂкгЩЩЯЪідРэЃЌдкЬњБэУцНјаа NH3 ЕФЗжНтЪЕбщЃЌЗЂЯжЗжНтЫйТЪгыХЈЖШЙиЯЕШчЭМЁЃДгЮќИНКЭНтЮќЙ§ГЬЗжЮіЃЌc0ЧАЫйТЪдіМгЕФдвђПЩФмЪЧ_________________________ЃЛc0 КѓЫйТЪНЕЕЭЕФдвђПЩФмЪЧ___________________ЁЃ
(4)вбжЊвКАБжаДцдкЃК2NH3(l)NH2Ѓ+NH4+ЁЃгУ Pt ЕчМЋЖдвКАБНјааЕчНтвВПЩВњЩњ H2КЭ N2ЁЃвѕМЋЕФЕчМЋЗДгІЪНЪЧ_______ЁЃ
ЁОД№АИЁП![]() ЃО ЃМ C АБЕФХЈЖШдіМгЃЌДпЛЏМСБэУцЮќИНЕФАБЗжзгдіЖрЃЌЫйТЪдіДѓ ДяЕНвЛЖЈХЈЖШКѓЃЌАБЗжзгХЈЖШЬЋДѓзшАN2КЭH2ЕФНтЮќ 2NH3 + 2eЃ= H2 + 2NH2Ѓ
ЃО ЃМ C АБЕФХЈЖШдіМгЃЌДпЛЏМСБэУцЮќИНЕФАБЗжзгдіЖрЃЌЫйТЪдіДѓ ДяЕНвЛЖЈХЈЖШКѓЃЌАБЗжзгХЈЖШЬЋДѓзшАN2КЭH2ЕФНтЮќ 2NH3 + 2eЃ= H2 + 2NH2Ѓ
ЁОНтЮіЁП
(1)вбжЊЂйNH3(l)NH3(g) ЁїH1ЃЌ
ЂкN2(g)+3H2(g)2NH3(l) ЁїH2ЃЌ
ИљОнИЧЫЙЖЈТЩПЩжЊ2ЁСЂй+ЂкПЩЕУЗДгІN2(g)+3H2(g)2NH3(g) ЁїH=2ЁїH1+ЁїH2ЃЛ
(2)ЂйвбжЊN2+3H22NH3ЃЌИУЗДгІЪЧЦјЬхЬхЛ§МѕаЁЕФЗДгІЃЌ ЫљвддкКубЙШнЦїЯрЕБгкКуШнШнЦїМгбЙЃЌЦНКте§ЯђвЦЖЏЃЌЫљвдКубЙШнЦїЂёжаNH3ЕФЬхЛ§ЗжЪ§ДѓгкКуШнШнЦїЂђЃЛ
ЂкЖдгкПЩФцЗДгІЃЌЕБЗДгІЮяЕФЭЖСЯБШЕШгкЗНГЬЪНжаМЦСПЪ§жЎБШЪБЃЌЩњГЩЮяжаЦјЬхЕФЬхЛ§ЗжЪ§еМБШзюДѓЃЌдђЕБ1mol N2КЭ3mol H2жУгкКуШнШнЦїжаЗДгІЪБЃЌАБЦјЕФЬхЛ§еМБШзюДѓЃЌИљОнЬтФППЩжЊДЫЪБNH3 ЕФЬхЛ§ЗжЪ§ЮЊ10%ЃЌЫљвдБЃГжЦфЫћЬѕМўВЛБфЃЌЦ№ЪМЪБИФЮЊГфШы2 mol N2КЭ2molH2ЃЌДяаТЦНКтКѓЃЌNH3ЕФЬхЛ§ЗжЪ§аЁгк10%ЃЛ
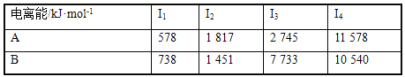
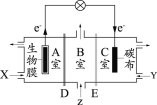
(3)ЂйгЩгкЛЏбЇМќЕФЖЯСбвЊЮќЪеФмСПЃЌЙЪЛюЛЏзДЬЌBЕФФмСПИпгкГѕЪМзДЬЌAЕФФмСПЃЌЖјДЫЗДгІЮЊЗХШШЗДгІЃЌЙЪГѕЪМзДЬЌAЕФФмСПИпгкФЉЬЌCЕФФмСПЃЌЙЪCЕФФмСПзюЕЭЃЛ
Ђкc0ЧААБЕФХЈЖШдіМгЃЌДпЛЏМСБэУцЮќИНЕФАБЗжзгдіЖрЃЌЕМжТЗДгІЫйТЪМгПьЃЌc0КѓгЩгкАБЗжзгХЈЖШЬЋДѓзшАЕЊЦјКЭЧтЦјЕФНтЮќЃЌЙЪЗДгІЫйТЪМѕТ§ЃЛ
(4)ЕчНтГижавѕМЋЕУЕчзгЗЂЩњЛЙдЗДгІЃЌИУЕчНтГижавѕМЋЩЯвКАБЛђяЇИљРызгЕУЕчзгЗЂЩњЛЙдЗДгІЃЌЕчМЋЗДгІЮЊ2NH3+2e-=H2+2NH2- ЁЃ

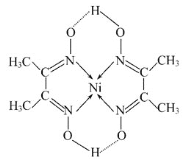
ЁОЬтФПЁПРћгУЬЋбєФмЁЂЗчФмЁЂЩњЮяжЪФмЕШПЩдйЩњФмдДЃЌзЊЛЏРћгУЖўбѕЛЏЬМЩшМЦГіЪЪКЯИпаЇЧхНрЕФКЯГЩШМСЯЗжзгНсЙЙЃЌЪЕЯжCO2+H2OЁњCxHyЕФЗжзгзЊЛЏЃЌЩњВњКЯГЩМзЭщЁЂДМУбШМСЯЁЂЭщЬўВёгЭЁЂКНПеШМгЭЕШПЩдйЩњКЯГЩШМСЯЁЃвђДЫЖўбѕЛЏЬМЕФВЖМЏЁЂРћгУЪЧЮвЙњФмдДСьгђЕФвЛИіживЊеНТдЗНЯђЁЃ
(1)вЛЖЈЬѕМўЯТЃЌдкCO2гызуСПЬМЗДгІЫљЕУЦНКтЬхЯЕжаМгШыH2КЭЪЪЕБДпЛЏМСЃЌгаЯТСаЗДгІЗЂЩњЃК
CO(g)+3H2(g)![]() CH4(g)+H2O(g) ЁїH1=-206.2kJ/mol
CH4(g)+H2O(g) ЁїH1=-206.2kJ/mol
CO(g)+H2O(g)![]() CO2(g)+H2(g) H2
CO2(g)+H2(g) H2
ШєCO2бѕЛЏH2ЩњГЩ0.1molCH4(g)КЭвЛЖЈСПЕФH2O(g)ЃЌећИіЙ§ГЬжаЗХГіЕФШШСПЮЊ16.5kJЃЌдђЁїH2=__ЁЃ
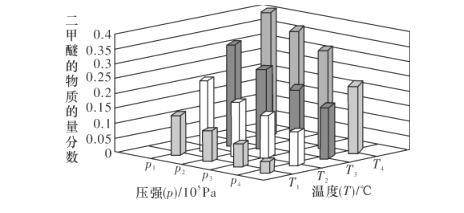
(2)КЯГЩЖўМзУбЕФзмЗДгІЮЊ2CO2(g)+6H2(g)![]() CH3OCH3(g)+3H2O(g) H=-122.4kJЁЄmol-1ЁЃФГЮТЖШЯТЃЌНЋ2.0molCO2(g)КЭ6.0molH2(g)ГфШыШнЛ§ЮЊ2LЕФУмБеШнЦїжаЃЌЗДгІЕНДяЦНКтЪБЃЌИФБфбЙЧПКЭЮТЖШЃЌЦНКтЬхЯЕжаCH3OCH3(g)ЕФЮяжЪЕФСПЗжЪ§БфЛЏЧщПіШчЭМЫљЪОЃЌдђp1__(ЬюЁА>ЁБЁА<"ЛђЁА=ЁБЃЌЯТЭЌ)p2ЁЃШєT3ЁЂp3ЃЌT4ЁЂp4ЪБЦНКтГЃЪ§ЗжБ№ЮЊK3ЁЂK4дђK3__K4ЃЌT1ЁЂp1ЪБH2ЕФЦНКтзЊЛЏТЪЮЊ___ЁЃ(НсЙћБЃСєШ§ЮЛгааЇЪ§зж)
CH3OCH3(g)+3H2O(g) H=-122.4kJЁЄmol-1ЁЃФГЮТЖШЯТЃЌНЋ2.0molCO2(g)КЭ6.0molH2(g)ГфШыШнЛ§ЮЊ2LЕФУмБеШнЦїжаЃЌЗДгІЕНДяЦНКтЪБЃЌИФБфбЙЧПКЭЮТЖШЃЌЦНКтЬхЯЕжаCH3OCH3(g)ЕФЮяжЪЕФСПЗжЪ§БфЛЏЧщПіШчЭМЫљЪОЃЌдђp1__(ЬюЁА>ЁБЁА<"ЛђЁА=ЁБЃЌЯТЭЌ)p2ЁЃШєT3ЁЂp3ЃЌT4ЁЂp4ЪБЦНКтГЃЪ§ЗжБ№ЮЊK3ЁЂK4дђK3__K4ЃЌT1ЁЂp1ЪБH2ЕФЦНКтзЊЛЏТЪЮЊ___ЁЃ(НсЙћБЃСєШ§ЮЛгааЇЪ§зж)
(3)Яђ2LУмБеШнЦїжаМгШы2molCO2КЭ6molH2ЃЌдкЪЪЕБЕФДпЛЏМСзїгУЯТЃЌЯТСаЗДгІФмздЗЂНјааЃКCO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)ЁЃ
CH3OH(g)+H2O(g)ЁЃ
ЂйИУЗДгІЁїH__(ЬюЁА>ЁБЁА<ЁБЛђЁА=ЁБ)0ЁЃ
ЂкЯТСаа№ЪіФмЫЕУїДЫЗДгІДяЕНЦНКтзДЬЌЕФЪЧ__(ЬюзжФИДњКХ)ЁЃ
aЃЎЛьКЯЦјЬхЕФЦНОљЯрЖдЗжзгжЪСПБЃГжВЛБф
bЃЎ1molCO2ЩњГЩЕФЭЌЪБга3molH-HМќЖЯСб
cЃЎCO2ЕФзЊЛЏТЪКЭH2ЕФзЊЛЏТЪЯрЕШ
dЃЎЛьКЯЦјЬхЕФУмЖШБЃГжВЛБф
ЂлЩЯЪіЗДгІГЃгУCuOКЭZnOЕФЛьКЯЮязїДпЛЏМСЁЃЯрЭЌЕФЮТЖШКЭЪБМфЖЮФкЃЌДпЛЏМСжаCuOЕФжЪСПЗжЪ§ЖдCO2ЕФзЊЛЏТЪКЭCH3OHЕФВњТЪгАЯьЕФЪЕбщЪ§ОнШчЯТБэЫљЪОЃК
Іи(CuO)% | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
CH3OHЕФВњ | 25% | 30% | 35% | 45% | 50% | 65% | 55% | 53% | 50% |
CO2ЕФзЊЛЏТЪ | 10% | 13% | 15% | 20% | 35% | 45% | 40% | 35% | 30% |
гЩБэПЩжЊЃЌCuOЕФжЪСПЗжЪ§ЮЊ__ДпЛЏаЇЙћзюМбЁЃ
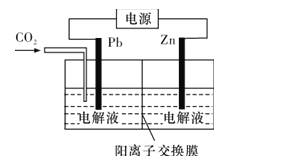
(4)CO2ПЩгУгкЙЄвЕжЦБИВнЫсаПЃЌЦфдРэШчЭМЫљЪО(ЕчНтвКВЛВЮМгЗДгІ)ЃЌZnЕчМЋЪЧ__МЋЁЃвбжЊдкPbЕчМЋЧјЕУЕНZnC2O4ЃЌдђPbЕчМЋЩЯЕФЕчМЋЗДгІЪНЮЊ__ЁЃ