��Ŀ����
����Ŀ����֪���¶ȵ���570 0C ʱ����ԭ������ˮ������Ӧ�IJ�����FeO������570 0Cʱ������Fe3O4����ʦ����ͼ��ʾʵ��װ�ã�����˻�ԭ������ˮ������Ӧ����ʾʵ�顣
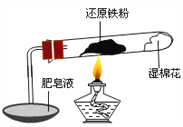
��ͬѧΪ̽��ʵ����Թ��ڵĹ��庬����Щ���ʣ�����������ʵ�飺
ʵ���� | ʵ����� | ʵ������ |
�� | ȡ������ɫ��ĩ�����Թ��У��������ᣬ�� | ��ɫ��ĩ���ܽ⣬��Һ��dz��ɫ�����������ݲ��� |
�� | ���Թ��еμӼ���KSCN��Һ���� | ��Һû�г���Ѫ��ɫ |
��������ʵ�飬����˵������ȷ���ǣ� ��
A. �Թ��ڵĹ���һ����������
B. �Թ��ڵĹ���һ��������Fe3O4
C. ����ȷ���Թ��ڵĹ���һ������FeO
D. ��ͨ�����Թ��ڹ��峹��ԭ��������������С�ķ�����ȷ���Ƿ���Fe3O4
���𰸡�B
����������ɫ��ĩ������������������ݲ���������һ���������ۣ�A��ȷ����ɫ��ĩ�������ۺ�Fe3O4��������������ܹ��������ӻ�ԭΪ�������ӣ����Բ���ȷ����ɫ��ĩ�Ƿ���Fe3O4��B�����������ᷴӦ������ð��˵��������������ȷ����ĩ���Ƿ���Fe3O4��FeO���������ᷴӦ������FeCl2�����������д���Fe3O4�������������ɵ�������������Ӧ�������������ӣ����Բ���ȷ���Թ��ڵĹ���һ������FeO��C��ȷ��FeO�к�������������Fe3O4�к�������������ͨ�����Թ��ڹ��峹��ԭ��������������С�ķ�����ȷ���Ƿ���Fe3O4�ǿ��е���D��ȷ����ȷѡ��B��

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�����Ŀ��������������A��B��C��D��E,������һ���Ǽ�,��������,����ˮ�������Բ����±��е�����:
������ | Na+��H+��Ba2+ |
������ | OH-�� CO32-��SO42- |
Ϊ��������,�ֱ��������ʵ��,��������:
��A��Һ��B��Һ��Ӧ������ɫ����X,����X������C��Һ��Ӧ���ɳ���E,����E����B��Һ��Ӧ;
��B��Һ��C��Һ��Ӧ���ɰ�ɫ����D,����D������ϡ���ᡣ
���������ʵ����,���:
(1)д�����ʵĻ�ѧʽ:A______ X______��
(2) B����ˮ��ĵ��뷽��ʽΪ______________________��
����B�������ӵ�ʵ�����������_____________________��
(3)д�� A��Һ��B��Һ��Ӧ�����ӷ���ʽ:
________________________________________________________________________��
(4)D��E�Ļ����a g,������������,��Ӧ������ɱ�״���µ�����b L,��D�ڻ�����е���������Ϊ________�����г���ʽ���ɣ�
����Ŀ���̷�(FeSO4��7H2O)������ȱ����ƶѪҩƷ����Ҫ�ɷ֡���������������м(����������������������)Ϊԭ�����������̷���һ�ַ�����
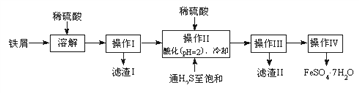
��ѯ���ϣ����й����ʵ��������±���
25��ʱ | ����H2S��Һ | SnS������ȫ | FeS��ʼ���� | FeS������ȫ |
pHֵ | 3.9 | 1.6 | 3.0 | 5.5 |
��1�������Ƶõ��̷��������Ƿ���Fe3+�����ѡ�õ��Լ�Ϊ____________________��
A.KSCN��ҺB.NaOH��ҺC.KMnO4��Һ
��2������II�У�ͨ�����������͵�Ŀ���ǣ�д���㣩___________��____________��
��3������IV��˳������Ϊ________����ȴ�ᾧ�����ˡ�
��4���ⶨ�̷���Ʒ��Fe2+�����ķ����ǣ�
a.��ȡ3.72g�̷���Ʒ���ܽ⣬��250mL����ƿ�ж��ݣ�
b.��ȡ25.00mL������Һ����ƿ�У�
c.�������ữ��0.01000mol/L KMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00mL��
�ٵζ�ʱʢ��KMnO4��Һ������Ϊ_________�����������ƣ���
�ڼ���������Ʒ��FeSO47H2O����������Ϊ__________��
�������ⶨ�У����ζ��ܹ��Ϊ50mL������a�г�ȡ��Ʒ���������ܳ���______g��(����4λС��)






