��Ŀ����
3����֪������A�ķ���ʽΪC4H6O2��������ˮ�������Է�����ͼ��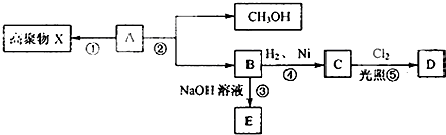 ͼ��ʾ�ı仯��
ͼ��ʾ�ı仯����֪��C���ʵ�һ�ȴ���Dֻ������ͬ���칹�壮��ش�
��1��A�����к��еĹ����ŵ�����̼̼˫����������
��2���٢ڢܢݷ�Ӧ������ȡ����Ӧ���Тڢݣ�����ţ���
��3��д���ڵķ�Ӧ����ʽCH2=CHCOOCH3+H2O
 CH2=CHCOOH+CH3OH��
CH2=CHCOOH+CH3OH����4��д��һ������Ҫ������ʵ�ͬ���칹��Ľṹ��ʽ
��C��ͬ���칹�������������ܷ���������ӦHCOOCH2CH3
��C��ͬ���칹�������ڴ����ܷ���������ӦCH3CHOHCHO��CH2OHCH2CHO��
��5��������֤D�к�����Ԫ�صķ���ȡ����D�����м�������������Һ��У��ټ���ϡ���������ԣ��μ���������Һ�����а�ɫ�������ɣ�֤��D�к�����Ԫ�أ�
��6��7.2gB��������̼��������Һ��Ӧ����״�������ɶ�����̼�����Ϊ2.24L��
���� ������A�ķ���ʽΪC4H6O2�����в����Ͷ�Ϊ2���������п�ͼ��֪��A�����ɼ״���B����A���������������һ��̼̼˫������B����3��̼ԭ�ӣ�����B��C����������֪��B����̼̼˫�������ݷ�Ӧ�ۣ���֪��B�����Ȼ���C���ʵ�һ�ȴ���Dֻ������ͬ���칹�壬����C�ĽṹΪCH3CH2COOH���ɴ˿��ƶϣ�BΪCH2=CHCOOH��EΪCH2=CHCOONa��AΪCH2=CHCOOCH3��A�����Ӿ۷�Ӧ��X��A����ˮ�⣨ȡ������Ӧ��B��B�����кͷ�Ӧ��E��B�����ӳɷ�Ӧ��C��C����ȡ����Ӧ����D���ݴ˴��⣮
��� �⣺������A�ķ���ʽΪC4H6O2�����в����Ͷ�Ϊ2���������п�ͼ��֪��A�����ɼ״���B����A���������������һ��̼̼˫������B����3��̼ԭ�ӣ�����B��C����������֪��B����̼̼˫�������ݷ�Ӧ�ۣ���֪��B�����Ȼ���C���ʵ�һ�ȴ���Dֻ������ͬ���칹�壬����C�ĽṹΪCH3CH2COOH���ɴ˿��ƶϣ�BΪCH2=CHCOOH��EΪCH2=CHCOONa��AΪCH2=CHCOOCH3��A�����Ӿ۷�Ӧ��X��A����ˮ�⣨ȡ������Ӧ��B��B�����кͷ�Ӧ��E��B�����ӳɷ�Ӧ��C��C����ȡ����Ӧ����D��
��1����������ķ�����֪��AΪCH2=CHCOOCH3��A�����к��еĹ����ŵ�����Ϊ̼̼˫�����������ʴ�Ϊ��̼̼˫����������
��2����������ķ�����֪���٢ڢܢݷ�Ӧ������ȡ����Ӧ���Тڢݣ��ʴ�Ϊ���ڢݣ�
��3����Ӧ�ڵķ�Ӧ����ʽΪCH2=CHCOOCH3+H2O CH2=CHCOOH+CH3OH���ʴ�Ϊ��CH2=CHCOOCH3+H2O
CH2=CHCOOH+CH3OH���ʴ�Ϊ��CH2=CHCOOCH3+H2O CH2=CHCOOH+CH3OH��
CH2=CHCOOH+CH3OH��
��4����C��ͬ���칹�������������ܷ���������Ӧ��Ϊ�����������Ľṹ��ʽΪHCOOCH2CH3���ʴ�Ϊ��HCOOCH2CH3��
��C��ͬ���칹�������ڴ����ܷ���������Ӧ��ΪCH3CHOHCHO��CH2OHCH2CHO���ʴ�Ϊ��CH3CHOHCHO��CH2OHCH2CHO��
��5��DΪC��һ�ȴ����֤D�к�����Ԫ�صķ���Ϊȡ����D�����м�������������Һ��У��ټ���ϡ���������ԣ��μ���������Һ�����а�ɫ�������ɣ�֤��D�к�����Ԫ�أ�
�ʴ�Ϊ��ȡ����D�����м�������������Һ��У��ټ���ϡ���������ԣ��μ���������Һ�����а�ɫ�������ɣ�֤��D�к�����Ԫ�أ�
��6��BΪCH2=CHCOOH����Ħ������Ϊ72g/mol��7.2gB��0.1mol��������̼��������Һ��Ӧ����״�������ɶ�����̼�����Ϊ0.1��22.4L=2.24L���ʴ�Ϊ��2.24��
���� ���⿼���л�����ƶϣ��л���ķ���ʽ������֮���ת��Ϊ�������ͻ�ƿڣ����ù����ŵı仯�����ƶϣ���Ŀ�Ѷ��еȣ�

| A�� | п���������������Ǹ��� | |
| B�� | п������ԭ��Ӧ������������������Ӧ | |
| C�� | ��Һ��OH-�������ƶ���K+��H+���ƶ� | |
| D�� | ���ŵ缫��Ӧ�IJ��Ͻ��У��������Һ��pH�������ֲ��� |
| A�� | FeS | B�� | CuS | C�� | CuCl2 | D�� | FeCl3 |
| A�� | ��ˮ�͵⻯�ط�Ӧ��Cl2+2I-=2Cl-+I2 | |
| B�� | ������AgNO3��Һ��Ӧ��HCl+Ag+=H++Ag Cl�� | |
| C�� | ����������Һ����������Cl2+2OH-=Cl-+ClO-+H2O | |
| D�� | �������̺�������������MnO2+4H++2Cl-$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��+2H2O |
| A�� | Cl2����H2S��Ӧ����S | |
| B�� | ����ˮʱHCl��ǿ�ᣬH2S������ | |
| C�� | ����ʱH2S�ֽܷ⣬HCl���� | |
| D�� | Cl2������Ӧ����FeCl3����S������Ӧ����FeS |
| A�� | ��LiCoO2��д��Ϊ���������ʽΪLi2O•Co2O3 | |
| B�� | �õ�صĵ��Һ����ʹ���л��ܼ���Ҫ�������л��ܼ������õĵ����� | |
| C�� | Li��3��Ԫ�أ�������Ľ���֮һ�����������ܱȸ� | |
| D�� | �ڵ�س�ŵ�ʱ��Li+�������缫֮������Ƕ�����Ƕ���õ�صij�ŵ練Ӧ����ʽΪ��LiCoO2+6C$?_{�ŵ�}^{���}$Li1-xCoO2+LixC6 |
| A�� | �ð�ˮ���չ�����SO2��2NH3•H2O+SO2=2NH4++SO32-+H2O | |
| B�� | Ca��HCO3��2 ��Һ�м�����������ʯ��ˮ��HCO3-+Ca2++OH-=CaCO3��+H2O | |
| C�� | FeI2��Һ��ͨ������Cl2��2Fe2++Cl2=2Fe3++2Cl- | |
| D�� | NaClO��Һ��FeCl2��Һ��ϣ�2ClO-+Fe2++2H2O=Fe��OH��2��+2HClO |
| A�� | 3�� | B�� | 6�� | C�� | 9�� | D�� | 10�� |
| A�� | 2-���� | B�� | 2��2-����-1-���� | ||
| C�� | ������ | D�� | 2-��-1-���� |