ћвƒњƒЏ»Ё
(1)ƒ≥ќё…Ђѕ°»№“ЇX÷–£ђњ…ƒ№Їђ”–ѕ¬±нЋщЅ–јл„”÷–µƒƒ≥ЉЄ÷÷°£
“хјл„” | CO32°™°ҐSiO32°™°ҐAlO2°™°ҐCl£≠ |
—фјл„” | Al3£Ђ°ҐCu2£Ђ°ҐMg2£Ђ°ҐNH4+°ҐNa£Ђ |
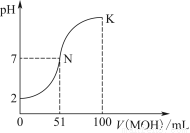
ѕ÷»°Є√»№“Ї Ѕњ£ђѕт∆д÷–Љ”»лƒ≥ ‘ЉЅY£ђ≤ъ…ъ≥Ѕµнµƒќп÷ µƒЅњ(n)”лЉ”»л ‘ЉЅYµƒћеїэ(V)µƒєЎѕµ»зЌЉЋщ Њ°£
Ґў»фY «—ќЋб£ђ‘т»№“Ї÷–Їђ”–µƒљр ф—фјл„” «_________________________________£ђ
abґќЈҐ…ъЈі”¶µƒ„№јл„”Јљ≥ћ љќ™___________________________________
±н÷–Oaґќ”лY»№“ЇЈі”¶µƒјл„”µƒќп÷ µƒЅњ÷Ѓ±»ќ™__________[“™±к√чјл„”ЈыЇ≈£ђ»зn(Na£Ђ)]°£
ҐЏ»фY «NaOH»№“Ї£ђ‘тbcґќЈі”¶µƒјл„”Јљ≥ћ љќ™______________________________
»ф≤їњЉ¬«јл„”µƒЋЃљв“тЋЎ£ђЇц¬‘H£ЂЇЌOH£≠µƒ”∞ѕм£ђ«“»№“Ї÷–÷їіж‘Џ4÷÷јл„”£ђ‘тЋь√«µƒјл„”Єц э±»ќ™___________________________________[∞і—фјл„”‘Џ«∞£ђ“хјл„”‘ЏЇу£ђЄяЉџ‘Џ«∞£ђµЌЉџ‘ЏЇуµƒЋ≥–т≈≈Ѕ–]°£
(2)ќэќ™µЏҐфA„е‘™ЋЎ£ђќэµƒµ•÷ ЇЌїѓЇѕќп”лƒ≥–©ќп÷ µƒїѓ—І–‘÷ …ѕ”––нґаѕаЋ∆÷Ѓі¶°£“—÷™ќэ‘™ЋЎЊя”–»зѕ¬–‘÷ £Ї
Sn4£Ђ£ЂSn=2Sn2£Ђ£ї
2Sn2£Ђ£ЂO2£Ђ4H£Ђ=2Sn4£Ђ£Ђ2H2O£ї
2H£Ђ£ЂSnO22°™ Sn(OH)2
Sn(OH)2 Sn2£Ђ£Ђ2OH£≠°£
Sn2£Ђ£Ђ2OH£≠°£
‘їЎір£Ї
Ґўќэ»№”Џ—ќЋб£ђ‘ўѕтЈі”¶Їуµƒ»№“Ї÷–Ќ®»л¬»∆ш£ђ”–єЎЈі”¶јаЋ∆”Џћъµƒѕа”¶±дїѓ£ђ ‘–і≥ц”–єЎЈі”¶µƒјл„”Јљ≥ћ љ£Ї______________________________________£ђ
ҐЏљЂҐў÷–»№“Ї’фЄ…ЇуЉћ–шЉ”»»Ћщµ√єћће£ђ±дїѓєэ≥ћјаЋ∆”ЏFeCl3»№“Їѕа”¶µƒ±дїѓ£ђ‘т„оЇуµ√µљµƒєћћеќп÷ «(Ј÷„” љ)__________°£
Ґџ»фњ…”√SnCl2»№“Ї”лєэЅњµƒЉо»№“ЇЈі”¶µƒЈљЈ®÷∆Sn(OH)2, Є√Љо «__________°£
(1)ҐўNa£Ђ°°CO32°™£Ђ2H£Ђ=H2O£ЂCO2°ь
n(SiO32°™)°√n(AlO2°™)£љ11°√2
ҐЏAl(OH)3£ЂOH£≠=AlO2°™£Ђ2H2O
N(Al3£Ђ)°√N(Mg2£Ђ)°√N(NH4+)°√N(Cl£≠)£љ2°√1°√4°√12
(2)ҐўSn£Ђ2H£Ђ=Sn2£Ђ£ЂH2°ь
Sn2£Ђ£ЂCl2=Sn4£Ђ£Ђ2Cl£≠
ҐЏSnO2°°ҐџNH3°§H2O
°Њљвќц°њ(1)Ґў»фYќ™—ќЋб£ђ‘тOaґќЈі”¶ќ™2H£Ђ£ЂSiO32°™=H2SiO3°э°ҐH£Ђ£ЂAlO2°™£ЂH2O=Al(OH)3°э£ђabґќЈі”¶ќ™2H£Ђ£ЂCO32°™=H2O£ЂCO2°ь£ђbcґќЈі”¶ќ™Al(OH)3£Ђ3H£Ђ=Al3£Ђ£Ђ3H2O£їX»№“Ї÷–Їђ”–AlO2°™32°™SiO32°™£ђє —фјл„”÷–÷ї”–Na£Ђњ…“‘іж‘Џ£ї…иbcґќЈі”¶ѕыЇƒµƒH£Ђµƒќп÷ µƒЅњќ™x£ђ‘тЄщЊЁbcґќЈі”¶њ…µ√£Їn(AlO2°™)£љ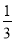 x£ђЄщЊЁOaґќЈі”¶њ…µ√£Їn(AlO2°™)£Ђ2n(SiO32°™)£љ4x£ђљвµ√n(AlO2°™)°√n(SiO32°™)£љ2°√11°£ҐЏ»фYќ™NaOH»№“Ї£ђ‘тOaґќЈі”¶ќ™Al3£Ђ£Ђ3OH£≠=Al(OH)3°э°ҐMg2£Ђ£Ђ2OH£≠=Mg(OH)2°э£ђabґќЈі”¶ќ™NH4+£ЂOH£≠??NH3°§H2O£ђbcґќЈі”¶ќ™Al(OH)3£ЂOH£≠=AlO2°™£Ђ2H2O£ї…иbcґќЈі”¶ѕыЇƒµƒOH£≠µƒќп÷ µƒЅњќ™y£ђ‘тЄщЊЁabґќЈі”¶њ…µ√n(NH4+)£љ2y£ђЄщЊЁbcґќЈі”¶њ…µ√n(AlO2°™)£љy£ђ‘тЄщЊЁ‘™ЋЎ ЎЇгњ…÷™n(Al3£Ђ)£љy£ђЄщЊЁOaґќЈі”¶њ…µ√3n(Al3£Ђ)£Ђ2n(Mg2£Ђ)£љ4y£ђљвµ√n(Mg2£Ђ)£љ
x£ђЄщЊЁOaґќЈі”¶њ…µ√£Їn(AlO2°™)£Ђ2n(SiO32°™)£љ4x£ђљвµ√n(AlO2°™)°√n(SiO32°™)£љ2°√11°£ҐЏ»фYќ™NaOH»№“Ї£ђ‘тOaґќЈі”¶ќ™Al3£Ђ£Ђ3OH£≠=Al(OH)3°э°ҐMg2£Ђ£Ђ2OH£≠=Mg(OH)2°э£ђabґќЈі”¶ќ™NH4+£ЂOH£≠??NH3°§H2O£ђbcґќЈі”¶ќ™Al(OH)3£ЂOH£≠=AlO2°™£Ђ2H2O£ї…иbcґќЈі”¶ѕыЇƒµƒOH£≠µƒќп÷ µƒЅњќ™y£ђ‘тЄщЊЁabґќЈі”¶њ…µ√n(NH4+)£љ2y£ђЄщЊЁbcґќЈі”¶њ…µ√n(AlO2°™)£љy£ђ‘тЄщЊЁ‘™ЋЎ ЎЇгњ…÷™n(Al3£Ђ)£љy£ђЄщЊЁOaґќЈі”¶њ…µ√3n(Al3£Ђ)£Ђ2n(Mg2£Ђ)£љ4y£ђљвµ√n(Mg2£Ђ)£љ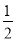 y£ђЄщЊЁµзЇ… ЎЇгњ…µ√n(Cl£≠)£љ6y£ђњ…µ√£Їn(Al3£Ђ)°√n(Mg2£Ђ)°√n(NH4+)°√n(Cl£≠)£љ2°√1°√4°√12°£(2)ҐўSnµƒЉтµ•—фјл„”ќ™Sn2£ЂЇЌSn4£Ђ£ђњ…«®“∆ћъ”л—ќЋбЈі”¶£ђ∆д≤ъќп‘ў”묻∆шЈі”¶µƒјл„”Јљ≥ћ љ£їҐЏFeCl3»№“Ї’фЄ…ЇуЉћ–шЉ”»»Ћщµ√єћћеќ™Fe2O3£ђє SnCl4»№“Ї’фЄ…ЇуЉћ–шЉ”»»Ћщµ√єћће”¶ќ™SnO2£їҐџ”…2H£Ђ£ЂSnO22°™
y£ђЄщЊЁµзЇ… ЎЇгњ…µ√n(Cl£≠)£љ6y£ђњ…µ√£Їn(Al3£Ђ)°√n(Mg2£Ђ)°√n(NH4+)°√n(Cl£≠)£љ2°√1°√4°√12°£(2)ҐўSnµƒЉтµ•—фјл„”ќ™Sn2£ЂЇЌSn4£Ђ£ђњ…«®“∆ћъ”л—ќЋбЈі”¶£ђ∆д≤ъќп‘ў”묻∆шЈі”¶µƒјл„”Јљ≥ћ љ£їҐЏFeCl3»№“Ї’фЄ…ЇуЉћ–шЉ”»»Ћщµ√єћћеќ™Fe2O3£ђє SnCl4»№“Ї’фЄ…ЇуЉћ–шЉ”»»Ћщµ√єћће”¶ќ™SnO2£їҐџ”…2H£Ђ£ЂSnO22°™ Sn(OH)2??Sn2£Ђ£Ђ2OH£≠њ…÷™Sn(OH)2Њя”–Ѕљ–‘£ђє ”¶”√»хЉоNH3°§H2OЇЌSnCl2Јі”¶÷∆»°Sn(OH)2“‘±№√в∆д”л«њЉоЈі”¶°£
Sn(OH)2??Sn2£Ђ£Ђ2OH£≠њ…÷™Sn(OH)2Њя”–Ѕљ–‘£ђє ”¶”√»хЉоNH3°§H2OЇЌSnCl2Јі”¶÷∆»°Sn(OH)2“‘±№√в∆д”л«њЉоЈі”¶°£

ƒ≥—Іѕ∞–°„й”ыƒ£ƒві”ƒ≥є§≥ІЈѕ“Ї÷–їЎ ’±ыЌ™°Ґ““іЉЇЌ““Ћбµƒ µ—й°£÷∆ґ®ЅЋ»зѕ¬ ‘—йЅч≥ћ°£
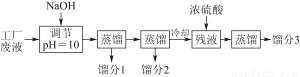
“—÷™Є√Јѕ“Ї÷–÷ч“™Їђ”–““іЉ£ђ∆д÷–їє»№”–±ыЌ™°Ґ““ЋбЇЌ““Ћб““х•°£«“Єч÷÷≥…Ј÷µƒЈ–µг»зѕ¬±н£Ї
ќп÷ | ±ыЌ™ | ““Ћб““х• | ““іЉ | ““Ћб |
Ј–µг£®°ж£© | 56.2 | 77.06 | 78 | 117.9 |
£®1£©ЅуЈ÷3µƒ≥…Ј÷ќ™____________°£
£®2£©…ѕ цЅч≥ћ÷–µчљЏpH£љ10µƒƒњµƒ «________________________________________________________________________________________________________________________________________________°£
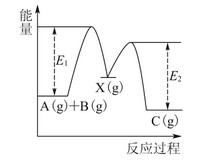
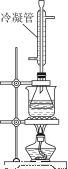
£®3£©Є√–°„йЌђ—Іµƒ’фЅу„∞÷√»зЌЉЋщ Њ°£‘тA÷–ќ¬ґ»Љ∆µƒќї÷√’э»Јµƒ «________£®ћо°∞a°±°∞b°±їт°∞c°±£©°£
£®4£©єъЉ“±к„Љєжґ®£ђ”≈÷ Єяґ»≈®ѕг–Ќ∞„Њ∆„№ЋбЅњ£®“‘““ЋбЉ∆£©”¶≤ї…ў”Џ0.30 g/L£ђ„№х•Ѕњ£®“‘““Ћб““х•Љ∆£©”¶≤ї…ў”Џ2.0 g/L°£
Ґўќ™≤вґ®ƒ≥∞„Њ∆—щ∆Јµƒ„№ЋбЅњ£ђ»°20.00 mL—щ∆Ј”Џ„ґ–ќ∆њ÷–£ђЉ”»лЈ”ћ™÷Є ЊЉЅ2µќ£ђ”√0.010 mol/LµƒNaOH±к„Љ»№“Їµќґ®÷Ѕ÷’µг°£≈–ґѕ÷’µгµƒ“јЊЁ «________________________________________________________________________________________________________________________________________________°£
»фЄ√∞„Њ∆—щ∆Јќ™”≈÷ Љґ£ђ‘тѕыЇƒNaOH»№“Їћеїэ”¶≤ї–°”Џ________mL°£
ҐЏ∞„Њ∆÷–µƒ„№х•Ѕњњ…”√ЈµµќЈ®≤вґ®°£Ќщ…ѕћвµќґ®Їуµƒ»№“Ї£®«°Ї√÷Ѕ÷’µг£©÷–‘ўЉ”»л20.00mL0.100mol/L NaOH±к„Љ»№“Ї£ђ”√ЌЉ„∞÷√ЋЃ‘°Љ”»»∞л–° ±°£јд»іЇу”√0.100mol/LµƒЅтЋб±к„Љ»№“Їµќґ®÷Ѕ÷’µг°£Љ”»»∞л–° ±µƒƒњµƒ «______________________£ђјдƒэє№µƒ„ч”√ «______________°£“—÷™„о÷’ѕыЇƒЅтЋб±к„Љ»№“Ї7.70 mL£ђЄ√∞„Њ∆—щ∆Ј÷–„№х•Ѕњќ™________g/L£®±£Ѕф–° эµгЇу»эќї э„÷£©°£
£®5£©ѕ¬Ѕ–≤ў„чїб є„№х•Ѕњ≤вґ®љбєы∆ЂЄяµƒ «________£®—°ћо±аЇ≈£©
a£ЃЉ”»» ±ќі є”√ЋЃ‘°ЇЌјдƒэє№
b£Ѓµќґ®«∞µќґ®є№ƒЏќё∆ш≈Ё£ђµќґ®Їу≤ъ…ъ∆ш≈Ё
c£Ѓµќґ®є№ќі”√ЅтЋб±к„Љ»№“Ї»уѕі