��Ŀ����
����Ŀ����Դ����������������ٵ��ش���⣬�״���δ����Ҫ����Դ����֮һ��
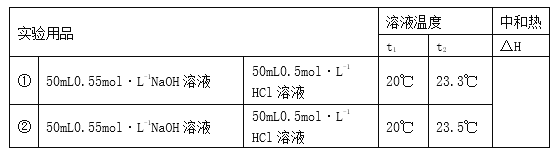
��1���ϳɼ״��ķ�ӦΪ��CO��g����2H2��g��![]() CH3OH��g������ͼ��ʾij�κϳ�ʵ������м״��������������CH3OH���뷴Ӧ�¶ȵĹ�ϵ���ߣ���÷�Ӧ����H__________0��������������������ͬ��
CH3OH��g������ͼ��ʾij�κϳ�ʵ������м״��������������CH3OH���뷴Ӧ�¶ȵĹ�ϵ���ߣ���÷�Ӧ����H__________0��������������������ͬ��

��2����ij�¶��£���һ���ݻ�������ܱ�������ͨ��2.5mol CO��7.5mol H2���ﵽƽ��ʱCO��ת����Ϊ90%����ʱ�����ڵ�ѹǿΪ��ʼʱ��__________����
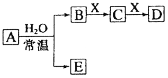
��3�����ü״�ȼ�ϵ�������ͼ��ʾ��װ�ã�

�����װ����bΪ____________����д��װ���е����ڷ�����Ӧ�����ӷ���ʽ_______________________��
����пƬ�������仯Ϊ12��8 gʱ��a�������ĵ�O2 �ڱ�״���µ����Ϊ___________L��
��4����̼�������Ե��ܺġ�����Ⱦ�����ŷ�Ϊ�����ľ���ģʽ������һ�ּ����ǽ�CO2ת�����л���ʵ��̼ѭ�����磺
2CO2��g����2H2O��l��= C2H4��g����3O2��g�� ��H��+1411��0 kJ/mol
2CO2��g����3H2O��l��= C2H5OH��1����3O2��g�� ��H��+1366��8 kJ/mol
������ϩˮ�����Ҵ���Ӧ���Ȼ�ѧ����ʽ____________________________________��
���𰸡��� 0��55 �� 2Cu2+��2H2O![]() 2Cu��O2����4H+ 2��24 C2H4��g����H2O��l��= C2H5OH ��H����44��2 kJ/mol
2Cu��O2����4H+ 2��24 C2H4��g����H2O��l��= C2H5OH ��H����44��2 kJ/mol
��������
��1���ϳɼ״��ķ�ӦΪ��CO��g����2H2��g��![]() CH3OH��g������ͼ��ij�κϳ�ʵ������м״��������������CH3OH���뷴Ӧ�¶ȵĹ�ϵ���߿�֪������CH3OH����230���Ժ����¶����߶���С��˵���÷�Ӧ�Ƿ��ȷ�Ӧ����÷�Ӧ����H��0��
CH3OH��g������ͼ��ij�κϳ�ʵ������м״��������������CH3OH���뷴Ӧ�¶ȵĹ�ϵ���߿�֪������CH3OH����230���Ժ����¶����߶���С��˵���÷�Ӧ�Ƿ��ȷ�Ӧ����÷�Ӧ����H��0��
��2����ij�¶��£���һ���ݻ�������ܱ�������ͨ��2.5mol CO��7.5mol H2���ﵽƽ��ʱCO��ת����Ϊ90%����CO�ı仯��Ϊ2.25mol��H2�ı仯��Ϊ4.5mol����ʱ������CO��g����H2��g����CH3OH�ĵ����ʵ����ֱ�Ϊ0.25mol��3mol��2.25mol����ͬ��ͬ����������У������ѹǿ֮�ȵ��������ʵ���֮�ȣ���ѹǿΪ��ʼʱ��![]() ����
����
��3����ȼ�ϵ���У�ȼ���ڸ�������������Ӧ����ͼ����Ϣ��֪���״���b�缫ͨ�룬���װ����bΪ��������ZnΪ�����������Ƕ��Ե缫���������ͭ��Һ�������ڷ�����Ӧ�����ӷ���ʽΪ2Cu2+��2H2O![]() 2Cu��O2����4H+��
2Cu��O2����4H+��
����пƬ�������仯Ϊ12��8 gʱ��˵��пƬ������ͭ�����ʵ���Ϊ0.2mol����a�������ĵ�O2 �����ʵ���Ϊ0.1mol�����ڱ�״���µ����Ϊ2.24L��
��4����2CO2��g����2H2O��l��= C2H4��g����3O2��g�� ��H��+1411��0 kJ/mol
��2CO2��g����3H2O��l��= C2H5OH��1����3O2��g�� ��H��+1366��8 kJ/mol
���ݸ�˹���ɣ��ɢ�-�ٿɵ�����ϩˮ�����Ҵ���Ӧ���Ȼ�ѧ����ʽC2H4��g����H2O��l��= C2H5OH ��H����44��2 kJ/mol��

����Ŀ���ճ������У�����β�����ŷŵ�NOx��CO��Ⱦ������������β��ϵͳ��װ�ô�ת����������Ч����NOx��CO���ŷš�
��֪:��2CO(g)+O2(g) ![]() 2CO2(g) ��H= -akJ/mol
2CO2(g) ��H= -akJ/mol
��N2(g)+O2(g) ![]() 2NO(g) ��H= +bkJ/mol
2NO(g) ��H= +bkJ/mol
��2NO(g)+O2(g) ![]() 2NO2(g) ��H= -ckJ/mol
2NO2(g) ��H= -ckJ/mol
�ش���������:
��1��CO��ȼ����Ϊ____________________��
��2��CO��NO2��ԭΪ���ʵ��Ȼ�ѧ����ʽΪ_____________________________��
��3��Ϊ��ģ�ⷴӦ2NO(g)+2CO(g) ![]() N2(g)+2CO2(g)�ڴ�ת�����ڵĹ������������һ���������÷�Ӧ�ں����ܱ������н��У��ô�������ò�ͬʱ��NO��CO��Ũ�����±�:
N2(g)+2CO2(g)�ڴ�ת�����ڵĹ������������һ���������÷�Ӧ�ں����ܱ������н��У��ô�������ò�ͬʱ��NO��CO��Ũ�����±�:
ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
c(NO)/(10-4mol/L) | 10.0 | 4.50 | 2.50 | 1.50 | 1.00 | 1.00 |
c(CO)/(10-3mol/L) | 3.60 | 3.05 | 2.85 | 2.75 | 2.70 | 2.70 |
��ǰ2s�ڵ�ƽ����Ӧ����V(N2)=_________________________________��
����˵��������Ӧ�ﵽƽ��״̬����_______________��
A��2n(CO2)=n(N2)
B����������ƽ����Է�����������
C�������ܶȲ���
D������������ѹǿ����
����NO��COŨ�����ʱ����ϵ��NO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
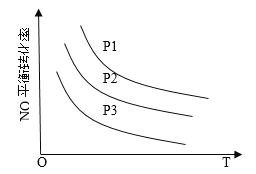
��NO��ƽ��ת�������¶����߶���С��ԭ����______________________________
ͼ��ѹǿ(P1��P2��P3)�Ĵ�С˳��Ϊ_____________________��