��Ŀ����
20�� ������Ҫ�ɷ�ΪNaAlSi2O6���������������ֽ��������ӣ�����Cr3+�ĺ�����������ɫ����dz��
������Ҫ�ɷ�ΪNaAlSi2O6���������������ֽ��������ӣ�����Cr3+�ĺ�����������ɫ����dz����1��Na��Al��Si��O����Ԫ�ص縺���ɴ�С��˳��ΪO��Si��Al��Na��
��2��Cr3+�Ļ�̬��������Ų�ʽΪ1s22s22p63s23p63d3��
��3��Cr���γ������K[Cr��C2O4��2��H2O��2]����H2O��Ϊ�ȵ������һ�ַ�����H2S���ѧʽ����ˮ��������ԭ�ӵ��ӻ���ʽΪsp3��1mol H2C2O4�����к��еĦҼ�����ĿΪ7mol��7��6.02��1023����
��4��Cr��Ca�����γ�һ�־���������Եĸ��������������ͼ��ʾ���ø���������Ļ�ѧʽ�ɱ�ʾΪCaCrO3��
���� ��1���ǽ�����Խǿ���縺��Խǿ��������Խ�����縺��Խǿ��
��2����24��Ԫ�أ���Ԫ��ʧȥ3�����ӱ��Cr3+������Cr3+������21�����ӣ����ݹ���ԭ��д�����������Ų�ʽ��
��3��ԭ������ͬ���۵�������ͬ�ķ���Ϊ�ȵ����壻����ˮ��������ԭ�ӵļ۲���Ӷ����жϣ������к���1���Ҽ���˫���к���һ���Ҽ���һ���м���
��4�����þ�̯����������ʵĻ�ѧʽ��
��� �⣺��1���ǽ�����Խǿ���縺��Խǿ��������Խ�����縺��Խǿ���ǽ����ԣ�O��Si��Al��Na����縺�ԣ�O��Si��Al��Na��
�ʴ�Ϊ��O��Si��Al��Na��
��2����24��Ԫ�أ���Ԫ��ʧȥ3�����ӱ��Cr3+������Cr3+������21�����ӣ����ݹ���ԭ��֪�������Ӻ�������Ų�ʽΪ��1s22s22p63s23p63d3��
�ʴ�Ϊ��1s22s22p63s23p63d3��
��3��ԭ������ͬ���۵�������ͬ�ķ���Ϊ�ȵ����壬����H2O��Ϊ�ȵ������һ�ַ�����H2S��ˮ��������ԭ�ӵļ۲���Ӷ���Ϊ$\frac{6+2}{2}$=4������Ϊsp3�ӻ���H2C2O4���Ӻ���2��O-H��2��C-O��2��C=O��1��C-C������7���Ҽ�������1mol H2C2O4�����к��еĦҼ�����ĿΪ 7mol��7��6.02��1023����
�ʴ�Ϊ��H2S��sp3��7mol��7��6.02��1023����
��4�����ݾ����ṹͼ�;�̯����֪��������Oԭ����Ϊ$\frac{1}{2}$��6=3��Caԭ����Ϊ$\frac{1}{8}$��8=1��Crԭ����Ϊ1����ѧʽΪCaCrO3��
�ʴ�Ϊ��CaCrO3��
���� ���⿼���Ϊ�ۺϣ���Ŀ�ѶȽϴ��漰�縺�Ե��жϡ������Ų�ʽ���ӻ����͵��жϡ��Ҽ�����Ŀ�ļ��㡢�����ļ���ȣ�ע�����þ�̯�����㾧���Ĺ��ɣ�ע�����û��ϼ۴�����Ϊ���ԭ����㣮

| A�� | ����ͨ������������Һ�� 2Cl2+2OH-�T3Cl-+ClO-+H2O | |
| B�� | ������ʯ��ʯ��Ӧ��2H++CO32-�TH2O+CO2�� | |
| C�� | п��ϡ���ᷴӦ��Zn+2H+�TZn2++H2�� | |
| D�� | ����ͭ��Һ������������Һ��Ӧ��Ba2++SO42-�TBaSO4�� |
| A�� | 0.2mol/L��AlCl3��Һ�У���������������0.2NA | |
| B�� | ��ҵ��ȡƯ��ʱ��������ÿ�Ƶ�25.4g��Ʒ����Ӧ��ת�Ƶ�����Ϊ0.2NA | |
| C�� | 30g���������30g�������к�C-H��������Ϊ2NA | |
| D�� | ��2.7g����Ͷ���������ȵ�Ũ�����г�ַ�Ӧ�����ռ������������6.72 L |
| A�� | ���ȷ�Ӧ�����ȷ�Ӧ�������� | |
| B�� | �����ڼ��Ȼ��ȼ�����½��еķ�Ӧ�������ȷ�Ӧ | |
| C�� | ��1L 1 mol/L NaOH��Һ�м���һ����ŨH2SO4��Һ��ǡ�÷�Ӧ��ȫʱ�����ų�65 kJ�����������ʾ�÷�Ӧ���к��ȵ��Ȼ�ѧ����ʽΪ��$\frac{1}{2}$H2SO4��aq��+NaOH��aq���T$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-65kJ•mol-1 | |
| D�� | ��֪��2CO��g��+O2��g���T2CO2��g����H=-566 kJ•mol-1���ɴ˿�֪CO��ȼ����Ϊ283 kJ•mol-1 |
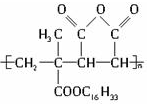 �����й�J��˵����ȷ���ǣ�������
�����й�J��˵����ȷ���ǣ�������| A�� | J����2�ֵ������۶��ɵ� | B�� | J����2�ֵ���Ӿ۶��ɵ� | ||
| C�� | J���п�ȼ�� | D�� | Jû�п�ȼ�� |
��NaOH��Һ�������Ƶ�Cu��OH��2����Һ���۵�ˮ��
| A�� | �� | B�� | �ڢ� | C�� | �٢ڢ� | D�� | �٢� |
| A�� | PCl3 | B�� | H2S | C�� | BeCl2 | D�� | SF6 |
| A�� | NH4+ | B�� | CO2 | C�� | SiH4 | D�� | CH4 |