��Ŀ����
1��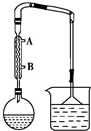 ij������ȤС����������Ϊ��Ҫԭ�ϣ�����ͼʾװ���Ʊ�1-�嶡�飮ʵ��������£�
ij������ȤС����������Ϊ��Ҫԭ�ϣ�����ͼʾװ���Ʊ�1-�嶡�飮ʵ��������£����Ʊ���Բ����ƿ�����μ���5mLˮ��5mLŨ���ᡢ3.1mL��������4.2g NaBr������ͼ��װ��Ӧװ�ã���С����Ȼ���������ڣ����ֻ���30min���ڴ˹�����Ҫ����ҡ����Ӧƿ��
����룺��ȥ��Դ��������ȥ����װ�ã���װ�øij���ͨ����װ�ã����Ž�������������õ��ֲ�Ʒ��
���ᴿ���ֲ�Ʒ��ϴ�ӡ��������Ȳ������õ���Ʒ��
| ���� | ��ɫ��״̬ | �е㣨�棩 | �ܶȣ�g•mL-1�� | ˮ���� |
| ������ | ��ɫҺ�� | 117.2 | 0.8098 | ���� |
| 1-�嶡�� | ��ɫҺ�� | 101.6 | 1.2758 | ���� |
��1���Ʊ�װ���������ܵ���������������������������ˮ�Ľ�ˮ��ΪB���A����B������
��2�����Ȼ�����Ӧƿ�е����ϳʺ���ɫ����ԭ����2HBr+H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$Br2��+SO2��+2H2O���û�ѧ����ʽ��ʾ����
��3�����Ʊ�1-�嶡��ʱ���ܱ߷�Ӧ�����������ԭ����1-�嶡�����������ķе���С�����߷�Ӧ������������н϶���������ӷ�������ԭ�ϵ������ʣ�
��4��Ҫ��ȥ1-�嶡���к��е���������Br2���������������ʺϵ���C��
A��NaCl��������B��NaOH��������C��NaHSO3��������D��KCl��
���� ��1��������������1-�嶡��ķе��֪������ʱ��Щ�������ӷ���Ϊʹ��Ӧ���ܳ�ֽ��з�Ӧ����Ҫ��������������ͬʱҪ�����ɵ����嵼��������������ȴЧ���Ϻý�ˮ���жϣ�
��2��Ũ������������廯�������嵥�ʣ����������嵥�ʶ��ʺ���ɫ��
��3��������������1-�嶡��ķе��֪�����ǵķе����С������1-�嶡��ʱ������Ҳ���ӷ���
��4������Br2��NaHSO3����������ԭ��Ӧ���⣮
��� �⣺��1��������������1-�嶡��ķе��֪������ʱ��Щ�������ӷ���Ϊʹ��Ӧ���ܳ�ֽ��з�Ӧ����Ҫ��������������ͬʱҪ�����ɵ����嵼�������������ܵ���������������������������������ȴЧ���Ϻÿ�֪������ˮӦ�ô�B�˽��룬��������������Ч���ã�
�ʴ�Ϊ������������������B��
��2��Ũ������������廯�������嵥�ʣ����������嵥�ʶ��ʺ���ɫ����Ӧ����ʽΪ2HBr+H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$Br2��+SO2��+2H2O��
�ʴ�Ϊ��2HBr+H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$Br2��+SO2��+2H2O��
��3��������������1-�嶡��ķе��֪�����ǵķе����С������1-�嶡��ʱ������Ҳ���ӷ���֪�����Ʊ�1-�嶡��ʱ���ܱ߷�Ӧ�����������ԭ���ǣ�1-�嶡�����������ķе���С�����߷�Ӧ������������н϶���������ӷ�������ԭ�ϵ������ʣ�
�ʴ�Ϊ��1-�嶡�����������ķе���С�����߷�Ӧ������������н϶���������ӷ�������ԭ�ϵ������ʣ�
��4��NaCl��KCl����û�з�Ӧ�����ܳ�ȥ�壬Br2����Ӧ�����ڼ��������¼���Ҳ��ˮ�⣬���Բ�����NaOH���壬����NaHSO3����������ԭ��Ӧ��
��ѡC��
���� ���⿼�������������Ʊ����嶡�顢���ʵķ����ᴿ��ʵ�鷽����ƣ��Ƚ�ȫ�濼�黯ѧ����֪ʶ����Ŀ�Ķ����ϴ�ѧ��������������һ����Ҫ���Ƕ�ѧ���ۺ������Ŀ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ��3������Ԫ��������� | B�� | ��1��Ԫ����ȫΪ����Ԫ�� | ||
| C�� | ��15����̬�⻯��ΪRH3 | D�� | ��17��Ԫ�صĵ�һ��Ԫ�������� |
| A�� | ̼������Һ | B�� | �Ȼ�����Һ | C�� | ���ᱵ��Һ | D�� | �������Һ |
| A�� | �屽�е������嵥�ʣ�NaOH��Һ����Һ�� | |
| B�� | �������е����ᣨNaOH��Һ������ | |
| C�� | �Ҵ��е�ˮ����ʯ�ҡ����ˣ� | |
| D�� | ���к����������ӣ���ˮ�����ˣ� |
| A�� | ������ѹǿ����ʱ��仯 | B�� | V����X��=2V����Z�� | ||
| C�� | ������X��Y��Z��Ũ����� | D�� | �����������ܶȲ��� |
| A�� | ��ӦBaSO4��s��+4C��s���TBaS��s��+4CO��g���������²����Է����У�˵���÷�Ӧ�ġ�H��0 | |
| B�� | ��֪����ʱ�����������ܶȻ�����Ϊ2.6��10-39��Ϊ��ȥij����CuCl2��Һ���������Ȼ������ɼ���������ʽ̼��ͭ������Һ��pH��4��ʹFe3+ת��Ϊ����������������ʱ��Һ�е�c��Fe3+��Ϊ2.6��10-8 mol/L | |
| C�� | ��ͬ���ʵ���Ũ�ȵ�������Һ����NH4Al��SO4��2��NH4Cl ��NH3•H2O��c��NH4+���ɴ�С��˳���ǣ��٣��ڣ��� | |
| D�� | ��Cu��OH��2����Һ�м��뵨������ƽ�����ƣ�������ͭ���ܽ�Ȳ��� |
| A�� | ���������CO2��SO2��SiO2��Cl2O7 | |
| B�� | �������ȼ���������Ȫˮ��ˮ��������¯�� | |
| C�� | ͬ�������壺ʯī����ʯ����������� | |
| D�� | �ǵ���ʣ����ǡ����Ȼ�̼������������ |
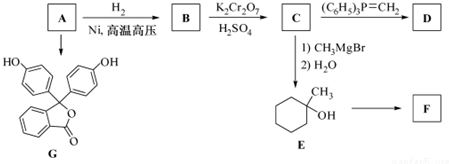
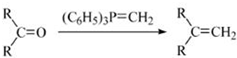

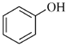 ��
��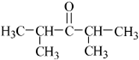 ��
��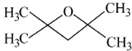 ����дһ�֣���
����дһ�֣���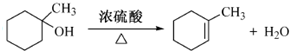 ��
��