题目内容
[2012·东北哈师大附中、东北师大附中、辽宁省实验中学第二次联合模拟](16分)某同学为了探究铜与浓硫酸的反应,进行了如下实验。
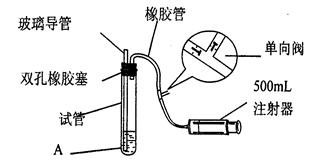
【实验1】铜与浓硫酸反应,实验装置如图所示。
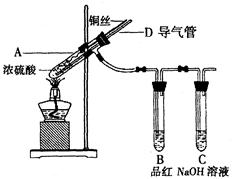
实验步骤:
①先连接好装置,检验气密性,加入试剂;
②加热A试管直到B中品红褪色,熄灭酒精灯;
③将铜丝向上提至离开液面。
(1)装置A中发生反应的化学方程式为 。
(2)熄灭酒精灯后,因为有导管D的存在,B中的液体不会倒吸,其原因是 。
(3)拆除装置前,不需打开胶塞,就可使装置中残留气体完全被吸收,应当采取的操是 。
【实验2】实验中发现试管内除了产生白色固体外,在铜丝表面还产生黑色固体甲,其中可能含有氧化铜、硫化铜、硫化亚铜,以及被掩蔽的氧化亚铜。
查阅资料:
①氧化亚铜在酸性环境下会发生自身氧化还原反应生成Cu2+和铜单质,在氧气流中煅烧,可以转化为氧化铜。
②硫化铜和硫化亚铜常温下都不溶于稀盐酸,在氧气流中煅烧,硫化铜和硫化亚铜都转化为氧化铜和二氧化硫。
为了研究甲的成分,该小组同学在收集到足够量的固体甲后,进行了如下图的实验:
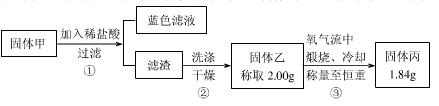
(4)②中检验滤渣是否洗涤干净的实验方法是 。
(5)③中在煅烧过程中一定发生的反应的化学方程式为 。
(6)下列对于固体甲的成分的判断中,正确的是(填字母选项) 。
【实验1】铜与浓硫酸反应,实验装置如图所示。

实验步骤:
①先连接好装置,检验气密性,加入试剂;
②加热A试管直到B中品红褪色,熄灭酒精灯;
③将铜丝向上提至离开液面。
(1)装置A中发生反应的化学方程式为 。
(2)熄灭酒精灯后,因为有导管D的存在,B中的液体不会倒吸,其原因是 。
(3)拆除装置前,不需打开胶塞,就可使装置中残留气体完全被吸收,应当采取的操是 。
【实验2】实验中发现试管内除了产生白色固体外,在铜丝表面还产生黑色固体甲,其中可能含有氧化铜、硫化铜、硫化亚铜,以及被掩蔽的氧化亚铜。
查阅资料:
①氧化亚铜在酸性环境下会发生自身氧化还原反应生成Cu2+和铜单质,在氧气流中煅烧,可以转化为氧化铜。
②硫化铜和硫化亚铜常温下都不溶于稀盐酸,在氧气流中煅烧,硫化铜和硫化亚铜都转化为氧化铜和二氧化硫。
为了研究甲的成分,该小组同学在收集到足够量的固体甲后,进行了如下图的实验:

(4)②中检验滤渣是否洗涤干净的实验方法是 。
(5)③中在煅烧过程中一定发生的反应的化学方程式为 。
(6)下列对于固体甲的成分的判断中,正确的是(填字母选项) 。
| A.固体甲中,CuS和Cu2S不能同时存在 |
| B.固体甲中,CuO和Cu2O至少有一种 |
| C.固体甲中若没有Cu2O,则一定有Cu2S |
| D.固体甲中可能有Cu2S |
(16分)(1)2H2SO4(浓)+Cu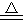 CuSO4+SO2↑+2H2O
CuSO4+SO2↑+2H2O
(2)试管A中气体压强减小,空气从D导管进入试管A中
(3)从D管口向A中大量鼓入空气
(4)取最后一次洗涤后所得液体于试管中,滴加硝酸银溶液,若无白色沉淀产生,则说明沉淀洗涤干净;若有白色沉淀生成,则说明未洗涤干净
(5)2CuS+3O2 2CuO+2SO2(条件也可为“煅烧”)
2CuO+2SO2(条件也可为“煅烧”)
(6)BCD
 CuSO4+SO2↑+2H2O
CuSO4+SO2↑+2H2O(2)试管A中气体压强减小,空气从D导管进入试管A中
(3)从D管口向A中大量鼓入空气
(4)取最后一次洗涤后所得液体于试管中,滴加硝酸银溶液,若无白色沉淀产生,则说明沉淀洗涤干净;若有白色沉淀生成,则说明未洗涤干净
(5)2CuS+3O2
 2CuO+2SO2(条件也可为“煅烧”)
2CuO+2SO2(条件也可为“煅烧”)(6)BCD
(6)由滤液呈蓝色可知,固体甲中含CuO和Cu2O至少有一种。煅烧CuS的反应方程式为2CuS+3O2 2CuO+2SO2,由该方程式可计算出2gCuS煅烧后固体质量减少0.33g;煅烧Cu2S的反应方程式为Cu2S+2O2
2CuO+2SO2,由该方程式可计算出2gCuS煅烧后固体质量减少0.33g;煅烧Cu2S的反应方程式为Cu2S+2O2 2CuO+SO2,该反应是固体质量不变的反应;煅烧Cu时固体质量增加,根据题意,固体甲中一定含有CuS,同时Cu2O和Cu2S至少一种。综上分析只有A项不正确。
2CuO+SO2,该反应是固体质量不变的反应;煅烧Cu时固体质量增加,根据题意,固体甲中一定含有CuS,同时Cu2O和Cu2S至少一种。综上分析只有A项不正确。
 2CuO+2SO2,由该方程式可计算出2gCuS煅烧后固体质量减少0.33g;煅烧Cu2S的反应方程式为Cu2S+2O2
2CuO+2SO2,由该方程式可计算出2gCuS煅烧后固体质量减少0.33g;煅烧Cu2S的反应方程式为Cu2S+2O2 2CuO+SO2,该反应是固体质量不变的反应;煅烧Cu时固体质量增加,根据题意,固体甲中一定含有CuS,同时Cu2O和Cu2S至少一种。综上分析只有A项不正确。
2CuO+SO2,该反应是固体质量不变的反应;煅烧Cu时固体质量增加,根据题意,固体甲中一定含有CuS,同时Cu2O和Cu2S至少一种。综上分析只有A项不正确。
练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目
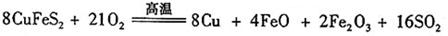
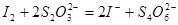 )
)