��Ŀ����
����Ŀ����������茶���![]() �ֳ�Ħ���Σ�
�ֳ�Ħ���Σ�![]() ��dz��ɫ������ˮ���ڿ����в��ױ�������ʵ�����Է���м�����������
��dz��ɫ������ˮ���ڿ����в��ױ�������ʵ�����Է���м�����������![]() �����۵����ʣ�Ϊ���Ʊ���������茶�����������£�
�����۵����ʣ�Ϊ���Ʊ���������茶�����������£�
�ش��������⣺
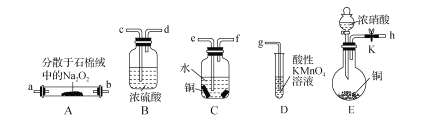
��1������������Ŀ����___________��
��2��������������ͼ��ʾװ������ɣ�����װ��ʡ�ԣ�������![]() ��������______����Ӧ�������г�������ζ�����������װ��
��������______����Ӧ�������г�������ζ�����������װ��![]() ��������_____________________��
��������_____________________��
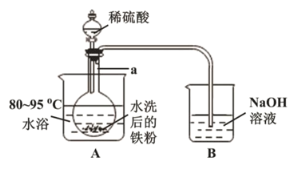
��3���������������������Һ���Ƿ���![]() ����ѡ���Լ�Ϊ_____________�����������Һ�в���
����ѡ���Լ�Ϊ_____________�����������Һ�в���![]() ��ԭ����______________________��������
��ԭ����______________________��������![]() ���Ǽ������ۣ�ָ���ò�������ҪĿ����________________��
���Ǽ������ۣ�ָ���ò�������ҪĿ����________________��
��4����Ʒ���Ȳⶨ����ȡ![]() ��������茶�����Ʒ�����������ܽ⣬������ƿ���Ƴ�
��������茶�����Ʒ�����������ܽ⣬������ƿ���Ƴ�![]() ��Һ����ȡ
��Һ����ȡ![]() ������Һ����ƿ�У�����
������Һ����ƿ�У�����![]() ����
����![]() ����Һ���Ϊ
����Һ���Ϊ![]() ��
��
�ٵζ����̷�Ӧ�����ӷ���ʽΪ_____________��
�ڲ�Ʒ�Ĵ���Ϊ_______________��
���𰸡���ȥ����м�������� Բ����ƿ ����![]() ����ֹ��Ⱦ
����ֹ��Ⱦ ![]() ��Һ ���ܹ����к��д����������ʣ�����
��Һ ���ܹ����к��д����������ʣ�����![]() �ܽ������
�ܽ������![]() ��Ӧ�� ��ֹ��Һ��
��Ӧ�� ��ֹ��Һ��![]() ������
������ ![]()
![]()
��������
����м���������![]() �����۵����ʣ�����м�����ȵ�̼������Һȥ���ۣ����˺�ϡ�������ܣ����˺����Һ���뱥���������Һ����������Ũ������ȴ�ᾧ�õ���������茶��塣
�����۵����ʣ�����м�����ȵ�̼������Һȥ���ۣ����˺�ϡ�������ܣ����˺����Һ���뱥���������Һ����������Ũ������ȴ�ᾧ�õ���������茶��塣
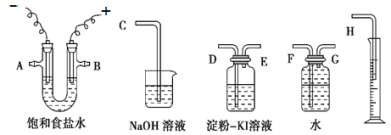
��1��̼������Һ�Լ��ԣ��ڼ��������£�����м�������۷���ˮ�⣬������Ŀ���dz�ȥ����м�������ۣ��ʴ�Ϊ����ȥ����м�������ۣ�
��2������![]() ������ΪԲ����ƿ����Ӧ�����У�����м���������������ᷴӦ�����������壬װ��
������ΪԲ����ƿ����Ӧ�����У�����м���������������ᷴӦ�����������壬װ��![]() �е�����������Һ�������ⷴӦ������װ��
�е�����������Һ�������ⷴӦ������װ��![]() ��������β������������
��������β������������![]() ����ֹ��Ⱦ���ʴ�Ϊ��Բ����ƿ������
����ֹ��Ⱦ���ʴ�Ϊ��Բ����ƿ������![]() ����ֹ��Ⱦ��
����ֹ��Ⱦ��
��3����Һ��![]() ��Һ�����Ƿ���
��Һ�����Ƿ���![]() ������Һ��ΪѪ��ɫ�������
������Һ��ΪѪ��ɫ�������![]() ������
������![]() ����ԭ�����ڡ����ܡ������к��д����������ʣ�����
����ԭ�����ڡ����ܡ������к��д����������ʣ�����![]() ��Ӧ���ɵ�
��Ӧ���ɵ�![]() ���������ۣ��ɷ���
���������ۣ��ɷ���![]() ������ҪĿ���Ƿ�ֹ
������ҪĿ���Ƿ�ֹ![]() ���������ʴ�Ϊ��
���������ʴ�Ϊ��![]() ��Һ�����ܹ����к��д����������ʣ�����
��Һ�����ܹ����к��д����������ʣ�����![]() �ܽ������
�ܽ������![]() ��Ӧ����ֹ��Һ��
��Ӧ����ֹ��Һ��![]() ��������
��������
��4���������������£��������ӱ�������������������ӷ�Ӧ����ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
�ڸ������ӷ�Ӧ����ʽ��֪��![]() ��
��![]() ��
��![]() �����Ʒ�Ĵ���Ϊ
�����Ʒ�Ĵ���Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
![]()

����Ŀ���Ե�Ʒλ����������Ҫ�ɷֿɱ�ʾΪ![]() ��
��![]() ��
��![]() ����������
����������![]() ��
��![]() ��
��![]() ��
��![]() ��Ϊԭ���Ʊ�����
��Ϊԭ���Ʊ�����![]() �Ĺ����������£�
�Ĺ����������£�
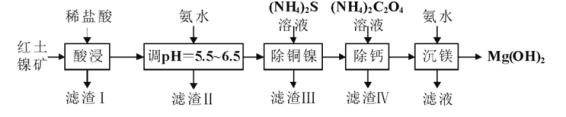
��֪��������IJ������±���
�������� | �ܶȻ� | ��ʼ���� | ��ȫ���� |
|
| 4.1 | 5.5 |
|
| 2.2 | 3.5 |
|
| 6.7 | 9.5 |
|
| 9.4 | 12.4 |
�ش��������⣺
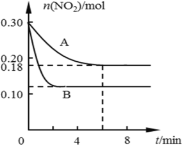
��1����������������Ҫ�ɷ���______���ѧʽ����
��2�������£���![]() ��Ŀ����______����
��Ŀ����______����![]() ʱ����Һ��
ʱ����Һ��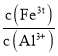 =______
=______
��3��д������ͭ�������̷�����Ӧ�����ӷ���ʽΪ![]() ��______________�������������̷�Ӧ�¶Ȳ��˳���
��______________�������������̷�Ӧ�¶Ȳ��˳���![]() ��ԭ��_________________________��
��ԭ��_________________________��
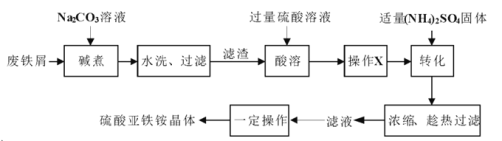
��4������Һ�������ʵ���Ҫ�ɷ�Ϊ________________����ȡ�����ʾ���ľ���ʵ�����Ϊ__________��