��Ŀ����
����Ŀ������㷺Ӧ����ϡ�н�����ʪ��ұ��ƯȾ��ҵ�������ӹ�����ҩƷ���л�ҩ��������������С�HCl ��������ˮ����ҵ���� HCl ��������ˮ�ķ�����ȡ���ᡣ
��1���� 12.0mol/L Ũ�������� 230mL 0.3mol/L ��ϡ���ᣬ��Ҫ��ȡŨ��������Ϊ___mL��
��2����Һϡ��������Ҫ�IJ����������ձ�������������Ͳ��____________��___________��
��3����Һϡ�����������²�����
a����ȡŨ�����һ�������ˮ�����ձ���ϡ��
b����������Ũ��������
c�����µߵ�ҡ��
d��������ˮ���̶��� 1-2cm �ط������ý�ͷ�ιܼ�����ˮ����Һ����̶�������
e����ϡ��Һת��������ƿ��ϴ���ձ��Ͳ�����������ϴ��Һת��������ƿ����
������ȷ�IJ���˳��Ϊ____________________________________________(�����)��
��4��ʵ������е����²����ᵼ������������ҺŨ�ȣ�����ƫ��������ƫС��������������
a����ȡŨ����ʱ���ӣ�______________________��
b����ȡŨ�������ϴ����Ͳ����ϴ��Һת��������ƿ��______________________��
c��ʵ��ǰ������ƿ����������������ˮ��______________________��
��5����״����1L ˮ��ͨ�� aL HCl ���壬����������Һ�� HCl �Ļӷ����õ���������Һ�ܶ�Ϊ b g/mL�����ʵ���Ũ��Ϊ ______________________mol/L��
���𰸡�6.3 250mL����ƿ ��ͷ�ι� b��a��e��d��c ƫС ƫ�� ���� ![]()
��������
(1)����������Һ���ѡ������ƿ�Ĺ����������Һϡ���������ʵ����ʵ������������ҪŨ����������
(2)��������һ�����ʵ���Ũ����Һһ�㲽��ѡ����Ҫ������
(3)����һ�����ʵ���Ũ����Һһ�㲽�裺���㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ����ǩ���ݴ�����
(4)�������������ʵ����ʵ�������Һ�����Ӱ�죬����c=![]() ������������
������������
(5)�ȸ���n=![]() �������״����aL�Ȼ�������ʵ�����Ȼ�����m=nM������Ȼ����������1Lˮ������ԼΪ1000g���Ӷ���֪��Һ�������ٸ���V=
�������״����aL�Ȼ�������ʵ�����Ȼ�����m=nM������Ȼ����������1Lˮ������ԼΪ1000g���Ӷ���֪��Һ�������ٸ���V=![]() �������Һ�������������c=
�������Һ�������������c=![]() ���������������ʵ���Ũ�ȡ�
���������������ʵ���Ũ�ȡ�
(1)��12.0mol/LŨ��������230mL 0.3mol/L��ϡ���ᣬӦѡ��250mL����ƿ������ҪŨ�������V����������Һϡ���������ʵ����ʵ����������ã�12.0mol/L��V=0.250L��0.3mol/L�����V=0.0063L=6.3mL���ʴ�Ϊ��6.3��
(2)����һ�����ʵ���Ũ����Һһ�㲽�裺���㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ��õ�����������Ͳ����ͷ�ιܡ��ձ���������������ƿ������230mL 0.3mol/L��ϡ���ᣬӦѡ��250mL�������ƿ����ȱ�ٵ�������250mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��250mL����ƿ����ͷ�ιܣ�
(3)����һ�����ʵ���Ũ����Һһ�㲽�裺���㡢��ȡ���ܽ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ����ǩ��������ȷ��˳��Ϊ��baedc���ʴ�Ϊ��baedc��
(4)a����ȡŨ����ʱ���ӣ�������ȡ��Ũ�������ƫС�����ʵ����ʵ���ƫС����ҺŨ��ƫС���ʴ�Ϊ��ƫС��
b����ȡŨ�������ϴ����Ͳ����ϴ��Һת��������ƿ��������ȡ��Ũ�������ƫ�����ʵ����ʵ���ƫ����ҺŨ��ƫ�ʴ�Ϊ��ƫ��
c��ʵ��ǰ������ƿ����������������ˮ�������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣻�ʴ�Ϊ�����䣻
(5)��״���µ�aL�Ȼ�����������ʵ���Ϊ��n(HCl)= ![]() =
=![]() mol����HCl��������36.5g/mol��
mol����HCl��������36.5g/mol��![]() mol=
mol=![]() g��1Lˮ������ԼΪ1000g�������������Ϊ��1000g+
g��1Lˮ������ԼΪ1000g�������������Ϊ��1000g+![]() g������������Ϊ��
g��������������![]() =
=![]() mL=
mL=![]() L�����Ը���������ʵ���Ũ��Ϊ��c(HCl)=
L�����Ը���������ʵ���Ũ��Ϊ��c(HCl)= 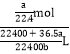 =
=![]() mol/L���ʴ�Ϊ��
mol/L���ʴ�Ϊ�� ![]() ��
��

 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�����Ŀ����������Ҫ�Ļ���ԭ�ϣ���ҽҩ��ұ�𡢻�����ʳƷ�����㷺ʹ�á�
I. �ô�����̼���ƹ�������500mL 0.40mol/L Na2CO3��Һ��
(1)��ȡNa2CO3�����������______________________g��
(2)������Һʱ���������²��������ղ���˳��4����_________(����ĸ)��
a. ���� b. ���� c. �ܽ� d. ҡ�� e. ת�� f. ϴ�� g. ����
(3)����˵���У���ȷ����_____________________(����ĸ)��
a. ����ʱ�����ӿ̶��ߣ��ᵼ�����Ƶ���ҺŨ��ƫС
b. ����ʱ�������ˮ�����̶��ߣ�Ҫ�õι�����
c. ת��ʱ����Һ��������ƿ�⣬Ҫ����������Һ
d. ҡ�Ⱥ�Һ����ڿ̶��ߣ�Ҫ�ټ�ˮ���̶���
II. ijʵ��С���ͬѧģ���°��Ƽ��ȡ����������£�

(1)��ҵ��������ĵ�һ���dz�ȥ����ʳ��ˮ����SO42�D��Ca2+���ӣ����μ�����Լ����������� ______________�� _______________�� (����)�� _______________��
(2)��֪�������ε��ܽ��
NaCl | NH4HCO3 | NaHCO3 | NH4Cl | |
�ܽ��(20��C��100gH2Oʱ) | 36.0 | 21.7 | 9.6 | 37.2 |
��д��װ��I�з�Ӧ�Ļ�ѧ����ʽ________________________________________��
��д��װ��II�з�����Ӧ�Ļ�ѧ����ʽ________________________________��
(3)�������п�ѭ�����õ�������__________________��
(4)�Ƴ��Ĵ�����ֻ��������NaCl��
�ټ����øô������Ƶ���Һ�к���Cl�D�ķ�����_________________________��
�ڲⶨ�ô���Ĵ��ȣ����з�������������__________(����ĸ)��
a. ��m�˴�����Ʒ�м�������CaCl2��Һ�����������ˡ�ϴ�ӡ������������Ϊb g
b. ��m�˴�����Ʒ�м�������ϡ���ᣬ�ü�ʯ��(��Ҫ�ɷ���CaO��NaOH)���ղ��������壬��ʯ������b g
c. ��m�˴�����Ʒ�м�������AgNO3��Һ�������ij��������ˡ�ϴ�ӡ������������Ϊb g






