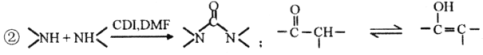��Ŀ����
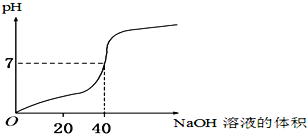
����Ŀ�������£���ijһԪ��HA��NaOH��Һ�������ϣ�ʵ����Ϣ���£�
ʵ���� | c(HA)/ mol��L��1 | c(NaOH)/ mol��L��1 | ��Ӧ����ҺpH |
�� | 0.1 | 0.1 | pH��9 |
�� | c1 | 0.2 | pH��7 |
�����жϲ���ȷ����
A.c1һ������0.2 mol��L��1
B.HA�ĵ��뷽��ʽ��HA![]() H����A��
H����A��
C.��Ӧ����Һ�У�c(Na��) �� c(OH��)�� c(A��) �� c(H��)
D.�ҷ�Ӧ����Һ�У�c(Na��) �� c(HA)��c(A��)
���𰸡�C
��������
��ʵ���֪����ͼ�ǡ�÷�Ӧ����Ӧ������pH��9��NaA��Һ��˵��A-����ˮ��ʹ��Һ�Լ��ԣ���HAΪ���ᡣ
A����Һ��һ������c��OH-��+c��A-��=c��Na+��+c��H+���������ҺpH=7˵����Һ�����ԣ�c��OH-��=c��H+��������c��A-��=c��Na+������Ӧ������NaA��Һ��A-����ˮ��ʹ��Һ�Լ��ԣ���HA��������ʹ��Һ�����ԣ���ˣ�ԭ��ҺŨ��c1��0.2 molL-1��A��ȷ��
B��HA��������ڵ���ƽ�⣬HA�ĵ��뷽��ʽ��HAH++A-��B��ȷ��
C����Ӧ������NaA��Һ��A-����ˮ���Լ��ԣ���Һ������Ũ�ȴ�Сc��Na+����c��A-����c��OH-����c��H+����C����
D������Һ��һ������c��OH-��+c��A-��=c��Na+��+c��H+���������ҺpH=7˵����Һ�����ԣ�c��OH-��=c��H+��������c��A-��=c��Na+����c��Na+����c��HA��+c��A-����D��ȷ��
��ѡC��

����Ŀ���û���̿��ԭ������������йط�ӦΪC(s)+2NO(g) ![]() N2(g)+CO2(g)��
N2(g)+CO2(g)��
��1��д��������Ӧ��ƽ�ⳣ������ʽ_______________��
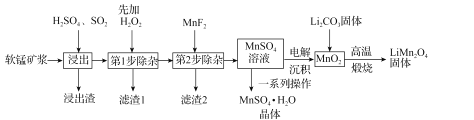
��2����2L�����ܱ����м�������C��NO������Ӧ����������������ش��������⡣
ʵ���� | �¶�/�� | ��ʼʱNO�����ʵ���/mol | ƽ��ʱN2�����ʵ���/mol |
1 | 700 | 0.40 | 0.09 |
2 | 800 | 0.24 | 0.08 |
����ϱ������ݣ��жϸ÷�Ӧ�ġ�H____0(����������������)��������_________��
���жϸ÷�Ӧ�ﵽƽ���������_______��
A.�����������ܶȺ㶨 B.�����ڸ�����Ũ�Ⱥ㶨
C.������ѹǿ�㶨 D.2v����NO��= v�棨N2��
��3��700��ʱ������2L����㶨���ܱ������г���һ����N2��CO2������Ӧ��N2(g)+CO2(g)![]() C(s)+2NO(g) ������N2��NO���ʵ�����ʱ��仯����������ͼ��ʾ����ش��������⡣
C(s)+2NO(g) ������N2��NO���ʵ�����ʱ��仯����������ͼ��ʾ����ش��������⡣
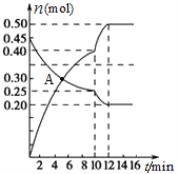
��0��10 min�ڵ�CO2ƽ����Ӧ����v��____________��
��ͼ��A��v(��)___v(��)�����������������������
�۵�10 minʱ�����ı������������_____________��
A���Ӵ��� B������C�����ʵ���
C����СCO2�����ʵ��� D������ E������