��Ŀ����
7���ֶ�±�ص���Cl2��Br2��I2��������ǿ������̽�����Ӷ��ܽ����Ԫ�����ڱ��У�ͬһ����Ԫ�طǽ����Ա仯���ɣ�������б��ó�����| ʵ�鲽�� | ʵ������ | ��Ӧ����ʽ |
| 1����������ˮ����ʢ��NaBr��Һ���Թ��У�����������������Ȼ�̼�������� | �ϲ�Ϊdz��ɫ������ɫ�� �²�Ϊ��ɫ | ���ӷ���ʽ�� Cl2+2Br-=Br2+2 Cl- |
| 1����������ˮ����ʢ��NaI��Һ���Թ��У�����������������Ȼ�̼�������� | �ϲ�Ϊdz��ɫ������ɫ�� �²�Ϊ�Ϻ�ɫ | ��ѧ����ʽ�� Br2+2NaI=I2+2NaBr |
��2��ͬһ����Ԫ�ش��ϵ��·ǽ�����������
���� 1�������������Դ����壬�����������ӷ���������ԭ��Ӧ�����壬������Ȼ�̼��Һ�ʳ�ɫ�����Ȼ�̼��ˮ�����ܣ����ܶȴ���ˮ���������Ȼ�̼���ܽ�ȴ���ˮ��
2����������Դ��ڵ⣬��KI��Һ�еμ��������������Һ����͵����ӷ����û���Ӧ���ɵ⣬�����������Ȼ�̼��ʹ����Һ����ɫ��
���ۣ���1������ʽ��������ǿ���û����������ģ�
��2�����ʵ�������Խǿ��Ԫ�صķǽ�����Խǿ��
��� �⣺1�������������Դ����壬�����������ӷ���������ԭ��Ӧ�����壬������Ȼ�̼��Һ�ʳ�ɫ�����Ȼ�̼��ˮ�����ܣ����ܶȴ���ˮ���������Ȼ�̼���ܽ�ȴ���ˮ�����Կ������������ϲ�Ϊdz��ɫ������ɫ�����²�Ϊ��ɫ�����ӷ���ʽΪCl2+2Br-=Br2+2 Cl-��
�ʴ�Ϊ���ϲ�Ϊdz��ɫ������ɫ�����²�Ϊ��ɫ��Cl2+2Br-=Br2+2 Cl-��
2����������Դ��ڵ⣬��KI��Һ�еμ��������������Һ����͵����ӷ����û���Ӧ���ɵ⣬�����������Ȼ�̼��ʹ����Һ����ɫ�����������������ȡ����KI��Һ���Թܣ����뼸�β�������µ���һ����Һ�����ּ���CCl4�������ã��������������ϲ�Ϊdz��ɫ������ɫ�����²�Ϊ�Ϻ�ɫ�����ӷ���ʽΪBr2+2NaI=I2+2NaBr��
�ʴ�Ϊ���ϲ�Ϊdz��ɫ������ɫ�����²�Ϊ�Ϻ�ɫ��Br2+2NaI=I2+2NaBr��
���ۣ���1������ʽ��������ǿ���û����������ģ���֪Cl2+2Br-=Br2+2 Cl-��Br2+2NaI=I2+2NaBr���������ԣ�Cl2��Br2��I2��
�ʴ�Ϊ��Cl2��Br2��I2��
��2�����ʵ�������Խǿ��Ԫ�صķǽ�����Խǿ����֪�����ԣ�Cl2��Br2��I2����ǽ����ԣ�Cl��Br��I������ͬһ����Ԫ�ش��ϵ��·ǽ�����������
�ʴ�Ϊ��������
���� ������±��Ԫ��Ϊ���忼��ͬһ����Ԫ�����ʵݱ���ɣ����ؿ���ѧ��ʵ��̽��������֪ʶ�����������ѵ���ʵ��������輰������������ѧ�������ȷ���ã���Ŀ�Ѷ��еȣ�

| A�� | SO2 | B�� | H2 | C�� | HCl | D�� | NH3 |
| A�� | KOH�к������Ӽ�Ҳ���й��ۼ����������ӻ����� | |
| B�� | HCl�д������Ӽ����������ӻ����� | |
| C�� | ���й��ۼ��Ļ�����һ���ǹ��ۻ����� | |
| D�� | ���������Ӽ�ͨ�������������γɵĻ�ѧ���������Ӽ� |
| A�� | v1��10v2 | |
| B�� | ������ˮϡ�ͣ���ˮ��$\frac{c��N{H}_{4}^{+}��}{c��N{H}_{3}•{H}_{2}O��}$����С | |
| C�� | v2��v3 | |
| D�� | ���A=B=C |
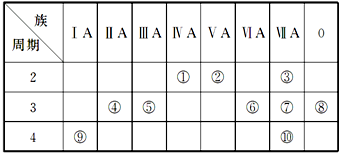
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� | �� |
��2���ܡ��ݡ�������Ԫ������������Ӧ��ˮ�����У�������ǿ����NaOH��
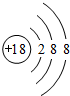
��3���ۡ�������Ԫ���γɵ����ӣ����Ӱ뾶�ɴ�С��˳����r��F-����r��Na+����
��4���ܺ͢�����Ԫ���γɻ�����Ļ�ѧʽΪNaBr���û�����ȼ��ʱ����ɫΪ��ɫ��
�û�������Һ��Ԫ�آ�ĵ��ʷ�Ӧ�����ӷ���ʽΪCl2+2Br-=2Cl-+Br2��
 ��
��

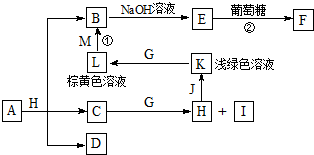 ������֮���ת����ϵ��ͼ������������ʡ�ԣ�C��D����X��Y��Z������Ԫ����ɵĻ����X��Y��Z��ԭ�������������������ڱ���X��ԭ�Ӱ뾶��С��Y��Zԭ������������֮��Ϊ10��DΪ��ɫ�ǿ�ȼ�����壬GΪ����ɫ�������壬J��MΪ������I��Ư�����ã���Ӧ�ٳ���������ӡˢ��·�壮
������֮���ת����ϵ��ͼ������������ʡ�ԣ�C��D����X��Y��Z������Ԫ����ɵĻ����X��Y��Z��ԭ�������������������ڱ���X��ԭ�Ӱ뾶��С��Y��Zԭ������������֮��Ϊ10��DΪ��ɫ�ǿ�ȼ�����壬GΪ����ɫ�������壬J��MΪ������I��Ư�����ã���Ӧ�ٳ���������ӡˢ��·�壮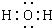 ��
��