��Ŀ����
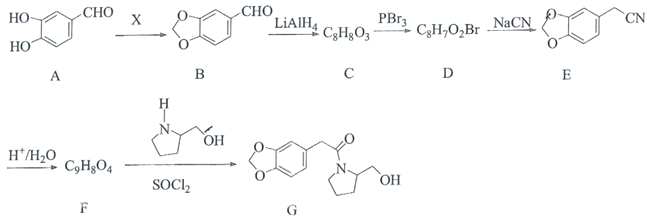
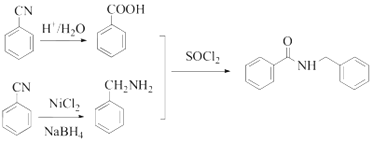
����Ŀ�����ϴ���¼���п��������������ã����м���G�ĺϳ���·��ͼ��
��֪��
��R-COOH+R��-NH2![]()
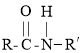 +H2O��
+H2O��
��R-CN![]()
![]()
�ش��������⣺
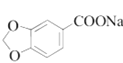
(1)XΪȩ�����ʣ�������Ϊ________��C�к��������ŵ�����Ϊ________��
(2)C��D�ķ�Ӧ����Ϊ________��
(3)������F�Ľṹ��ʽΪ________��
(4)B�����Ƶ�������ͭ����Һ��Ӧ�Ļ�ѧ����ʽΪ________��
(5)�л���Y��A��ͬ���칹�壬����������������![]() ��Һ������ɫ��Ӧ����
��Һ������ɫ��Ӧ����![]() ����������Na��Ӧ����
����������Na��Ӧ����![]() ���۽ṹ�к���
���۽ṹ�к���![]() ����Y����________�֣����к˴Ź���������ʾΪ4��壬�ҷ������Ϊ
����Y����________�֣����к˴Ź���������ʾΪ4��壬�ҷ������Ϊ![]() ������Ϊ________(д������һ�ֽṹ��ʽ)��
������Ϊ________(д������һ�ֽṹ��ʽ)��
(6)�����![]() Ϊ��ʼԭ���Ʊ�
Ϊ��ʼԭ���Ʊ�![]() �ĺϳ���·(���Լ���ѡ) ________��
�ĺϳ���·(���Լ���ѡ) ________��
���𰸡���ȩ �ǻ����Ѽ� ȡ����Ӧ ![]()
 +2Cu(OH)2+NaOH
+2Cu(OH)2+NaOH![]()
 +Cu2O��+3H2O 8
+Cu2O��+3H2O 8 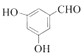 ��
�� ��
��![]()
��������
A��X��Ӧ����B�����A��B�Ľṹ��ʽ���ɵ�XΪ��ȩ��B��LiAlH4������������C��C����PBr3���Եõ�D��D�����軯�ƺ�õ�E���ữ��õ�F�����B��E�Ľṹ��ʽ����C��D�ķ���ʽ���ɵ�C�Ľṹ��ʽΪ![]() ��D�Ľṹ��ʽΪ
��D�Ľṹ��ʽΪ![]() ��F�Ľṹ��ʽΪ
��F�Ľṹ��ʽΪ![]() ��F��
��F��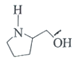 ��SOCl2����������G���ݴ˷������
��SOCl2����������G���ݴ˷������
(1) A��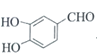 ��XΪȩ�����ʣ��õ�
��XΪȩ�����ʣ��õ� ������̼ԭ����Ŀ�ı仯����֪XΪ��ȩ������C�Ľṹ��֪C�к��������ŵ�����Ϊ�Ѽ����ǻ���
������̼ԭ����Ŀ�ı仯����֪XΪ��ȩ������C�Ľṹ��֪C�к��������ŵ�����Ϊ�Ѽ����ǻ���
(2) C�Ľṹ��ʽΪ![]() ��D�Ľṹ��ʽΪ
��D�Ľṹ��ʽΪ![]() ��C�е��ǻ���Brԭ��ȡ������Ӧ����Ϊȡ����Ӧ��
��C�е��ǻ���Brԭ��ȡ������Ӧ����Ϊȡ����Ӧ��
(3)���ݷ�����������F�Ľṹ��ʽΪ![]() ��
��
(4)B����ȩ�������л�ԭ�ԣ����Խ����Ƶ�������ͭ��ԭ��������ͭ��B�����Ƶ�������ͭ����Һ��Ӧ�Ļ�ѧ����ʽΪ +2Cu(OH)2+NaOH
+2Cu(OH)2+NaOH![]()
 +Cu2O��+3H2O��
+Cu2O��+3H2O��
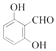
(5)�л���Y��A��ͬ���칹�壬����ʽΪC7H6O3��������������������Һ������ɫ��Ӧ�����з��ǻ��ͱ�������
![]() ����������Na��Ӧ����
����������Na��Ӧ����![]() �����Ժ����ǻ����Ȼ����ҷ����к����ǻ����Ȼ���2�������ṹ�к���
�����Ժ����ǻ����Ȼ����ҷ����к����ǻ����Ȼ���2�������ṹ�к���![]() ����������ȩ�����Ȼ��Ľṹ����Y���ӽṹ���������ǻ���һ��ȩ���뱽������������5�ֽṹ����Y���ӵĽṹ��һ�����ǻ���һ���Ȼ��뱽��������ȡ�������ڣ��䣬�Ե�λ�ã���3�֣���Y�Ľṹ����8�֣����к˴Ź���������ʾΪ4��壬�ҷ������Ϊ
����������ȩ�����Ȼ��Ľṹ����Y���ӽṹ���������ǻ���һ��ȩ���뱽������������5�ֽṹ����Y���ӵĽṹ��һ�����ǻ���һ���Ȼ��뱽��������ȡ�������ڣ��䣬�Ե�λ�ã���3�֣���Y�Ľṹ����8�֣����к˴Ź���������ʾΪ4��壬�ҷ������Ϊ![]() ������Ϊ
������Ϊ

![]() �е�����һ�֣�
�е�����һ�֣�
(6)��![]() Ϊ��ʼԭ���Ʊ�
Ϊ��ʼԭ���Ʊ�![]() �ĺϳ���·Ϊ
�ĺϳ���·Ϊ ��
��

 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д� �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�����Ŀ�����ᶡ������Ҫ�Ļ���ԭ�ϡ�ʵ���������ᡢ������Ũ�����������������������Ʊ����ᶡ����װ��ʾ��ͼ(���Ⱥͼг�װ����ʡ��)���й���Ϣ���£�
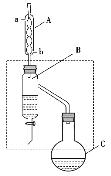
���� | ������ | ���ᶡ�� | |
�۵�/�� | 16.6 | -89.5 | -73.5 |
�е�/�� | 117.9 | 117 | 126.0 |
�ܶ�/g��cm-3 | 1.1 | 0.80 | 0.88 |
����˵����ȷ���ǣ� ��
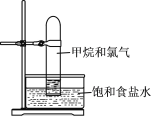
A.����һ��ʱ�������ƿC�����Ǽӷ�ʯ���ɴ�ƿ��ֱ�Ӽ��뼴��
B.װ��B�������Dz��Ϸ�������ᶡ������߲���
C.װ��A����װ������װ�õ���������������ˮ��a�ڽ���b�ڳ�
D.���ᶡ���в�������������������ñ���̼������Һ��ȥ