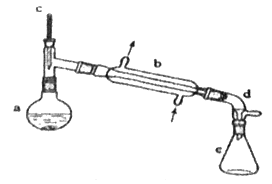
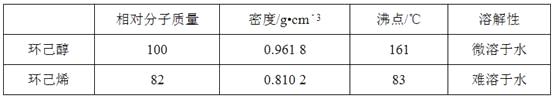
��Ŀ����
����Ŀ����������������ʱ���������CO��NOx�ȶ�����Ⱦ���壬��δ�����Щ���壬�Ա����������������شش���������:
��1����֪��2NO2(g) ![]() 2NO(g)��O2(g)����H1����115.2 kJ��mol��1��
2NO(g)��O2(g)����H1����115.2 kJ��mol��1��
2O3(g) ![]() 3O2(g)����H2����286.6 kJ��mol��1��
3O2(g)����H2����286.6 kJ��mol��1��
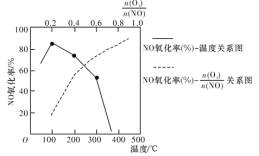
д��������NO���ò���NO2��O2���Ȼ�ѧ����ʽ��___________�������ܱ���ϵ��NO��������![]() ֵ�ı仯�Լ����¶ȵı仯������ͼ��ʾ��NO��������
ֵ�ı仯�Լ����¶ȵı仯������ͼ��ʾ��NO��������![]() ֵ������������Ҫԭ����________________________��
ֵ������������Ҫԭ����________________________��
��2��ʵ���÷�Ӧ2NO(g)+O2(g) ![]() 2NO2(g) ��H<0�ļ�ʱ��Ӧ�����������¹�ϵʽ��v��=k����c2(NO)��c(O2)��v��=k����c2(NO2)��k����k��Ϊ���ʳ��������¶�Ӱ��
2NO2(g) ��H<0�ļ�ʱ��Ӧ�����������¹�ϵʽ��v��=k����c2(NO)��c(O2)��v��=k����c2(NO2)��k����k��Ϊ���ʳ��������¶�Ӱ��
���¶�ΪT1ʱ����1L�ĺ����ܱ������У�Ͷ��0.6 molNO��0.3 molO2�ﵽƽ��ʱO2Ϊ0.2 mol���¶�ΪT2ʱ���÷�Ӧ����k��=k������T1_______ T2 (��������������С��������������)��
���о����ָ÷�Ӧ�����²�����У�
��һ����NO+NO![]() N2O2 ����ƽ�� �ڶ�����N2O2 +O2
N2O2 ����ƽ�� �ڶ�����N2O2 +O2![]() 2NO2 ����Ӧ
2NO2 ����Ӧ
���пɽ�����Ϊ�ڶ�����Ӧ��Ӱ���һ����ƽ�⣬��һ����Ӧ�У�v1��=k1����c2(NO)��v1��=k1����c(N2O2)
����������ȷ����______
A.ͬһ�¶��£�ƽ��ʱ��һ����Ӧ��![]() Խ��Ӧ����̶�Խ��
Խ��Ӧ����̶�Խ��
B.�ڶ�����Ӧ���ʵͣ����ת����Ҳ��
C.�ڶ����Ļ�ܱȵ�һ���Ļ�ܵ�
D.������Ӧ�������ɵڶ�����Ӧ���ʾ���
��3����ѧ���о�����һ�ָ�Ч���������Խ�CO��NO2����ת��Ϊ����Ⱦ���壬��Ӧ����ʽΪ��2NO2(g)+4CO(g) ![]() 4CO2(g)+N2(g) ��H<0
4CO2(g)+N2(g) ��H<0
ij�¶��£���10L�ܱ������зֱ����0.1molNO2��0.2 molCO������������Ӧ�����ŷ�Ӧ�Ľ��У������ڵ�ѹǿ�仯���±���ʾ��
ʱ��/min | 0 | 2 | 4 | 6 | 8 | 10 | 12 |
ѹǿ/kPa | 75 | 73.4 | 71.95 | 70.7 | 69.7 | 68.75 | 68.75 |
�ش��������⣺
���ڴ��¶��£���Ӧ��ƽ�ⳣ��Kp=_________kPa-1(KpΪ�Է�ѹ��ʾ��ƽ�ⳣ������������ȷ��С�����2λ)���������¶Ȳ��䣬�ٽ�CO��CO2����Ũ�ȷֱ�����һ������ƽ��_____(���������������������������ƶ���)��
�������¶Ƚ��ͣ��ٴ�ƽ�����ԭƽ�������ϵѹǿ(p��)��α仯��_______(����������������С������������)��ԭ����_____________________��
���𰸡�NO(g)��O3(g)![]() NO2(g)��O2(g)����H����200.9 kJ��mol��1
NO2(g)��O2(g)����H����200.9 kJ��mol��1 ![]() ֵ����O3Ũ�����ӣ�������ƽ��NO(g)��O3(g)
ֵ����O3Ũ�����ӣ�������ƽ��NO(g)��O3(g)![]() NO2(g)��O2(g)�����ƶ���NO���������� С�� AD 0.04 ���ƶ� ��С ���¶Ƚ��ͣ�������䣬���ݰ����ӵ����ɣ���ѹǿ��С��ͬʱ�������¶ȣ����ڷ�Ӧ���ȣ�����ƽ�⳯�������ƶ��������ڷ��������٣���ѹǿҲ��С
NO2(g)��O2(g)�����ƶ���NO���������� С�� AD 0.04 ���ƶ� ��С ���¶Ƚ��ͣ�������䣬���ݰ����ӵ����ɣ���ѹǿ��С��ͬʱ�������¶ȣ����ڷ�Ӧ���ȣ�����ƽ�⳯�������ƶ��������ڷ��������٣���ѹǿҲ��С
��������
(1)���ݸ�˹������д������NO���ò���NO2��O2���Ȼ�ѧ����ʽ��O3Ũ�����ӣ�������ƽ��NO(g)��O3(g)![]() NO2(g)��O2(g)�����ƶ���
NO2(g)��O2(g)�����ƶ���
(2) 2NO(g)+O2(g)![]() 2NO2(g)��v��=k����c2(NO)��c(O2)��v��=k����c2(NO2)���ﵽƽ��ʱk����c2(NO)��c(O2)= k����c2(NO2)��
2NO2(g)��v��=k����c2(NO)��c(O2)��v��=k����c2(NO2)���ﵽƽ��ʱk����c2(NO)��c(O2)= k����c2(NO2)��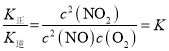 ��
��
(3)�����������ʵ����ȵ���ѹǿ�ȣ�![]() ��x=0.275mol������Ӧƽ��ʱ�����������ʵ�����0.275mol��
��x=0.275mol������Ӧƽ��ʱ�����������ʵ�����0.275mol��
(1)��2NO2(g)![]() 2NO(g)��O2(g)����H1����115.2 kJ��mol��1��
2NO(g)��O2(g)����H1����115.2 kJ��mol��1��
��2O3(g)![]() 3O2(g)����H2����286.6 kJ��mol��1��
3O2(g)����H2����286.6 kJ��mol��1��
���ݸ�˹���ɣ�����![]() ������
������![]() �ã�NO(g)��O3(g)
�ã�NO(g)��O3(g)![]() NO2(g)��O2(g)����H����200.9 kJ��mol��1��
NO2(g)��O2(g)����H����200.9 kJ��mol��1��![]() ֵ����O3Ũ�����ӣ�������ƽ��NO(g)��O3(g)
ֵ����O3Ũ�����ӣ�������ƽ��NO(g)��O3(g)![]() NO2(g)��O2(g)�����ƶ���NO����������
NO2(g)��O2(g)�����ƶ���NO����������
(2)���¶�ΪT1ʱ����1L�ĺ����ܱ������У�Ͷ��0.6 molNO��0.3 molO2�ﵽƽ��ʱO2Ϊ0.2 mol��

�¶�ΪT1ʱ��K= ���¶�ΪT2ʱ���÷�Ӧ����k��=k������K=1��2NO(g)+O2(g)
���¶�ΪT2ʱ���÷�Ӧ����k��=k������K=1��2NO(g)+O2(g)![]() 2NO2(g)����Ӧ���ȣ�����T1С��T2��
2NO2(g)����Ӧ���ȣ�����T1С��T2��
��A��![]() ��ͬһ�¶��£�ƽ��ʱ��һ����Ӧ��
��ͬһ�¶��£�ƽ��ʱ��һ����Ӧ��![]() Խ��˵��ƽ�ⳣ��Խ��Ӧ����̶�Խ��A��ȷ��
Խ��˵��ƽ�ⳣ��Խ��Ӧ����̶�Խ��A��ȷ��
B��ת������ƽ���ƶ��йأ��뷴Ӧ�����أ���B����
C�����ԽС����Ӧ����Խ�죬�ڶ����Ļ�ܱȵ�һ���Ļ�ܸߣ���C����
D���ܷ�Ӧ����������Ӧ�������ڶ�����Ӧ������������������Ӧ�������ɵڶ�����Ӧ���ʾ�������D��ȷ��
(3) ��
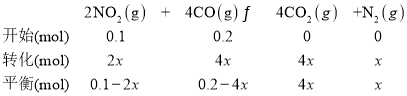
![]()
x=0.025mol��
�ڴ��¶��£���Ӧ��ƽ�ⳣ��Kp=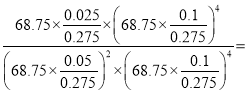 0.04kPa-1���������¶Ȳ��䣬�ٽ�CO��CO2����Ũ�ȷֱ�����һ����Ũ����Q=k����ƽ�ⲻ�ƶ���
0.04kPa-1���������¶Ȳ��䣬�ٽ�CO��CO2����Ũ�ȷֱ�����һ����Ũ����Q=k����ƽ�ⲻ�ƶ���
�����¶Ƚ��ͣ�������䣬���ݰ����ӵ����ɣ���ѹǿ��С��ͬʱ�������¶ȣ����ڷ�Ӧ���ȣ�����ƽ�⳯�������ƶ��������ڷ��������٣���ѹǿҲ��С�����������¶Ƚ��ͣ��ٴ�ƽ�����ԭƽ�������ϵѹǿ(p��)��С��

����Ŀ�������������������ܺͽ����ܵ�ԭ�ϡ�һ�����ú��ܷ���(��Ҫ�ɷ�ΪCo2O3��������Ni��Fe��Al2O3��CaO��̿���л����)��ȡCoC2O4�Ĺ����������£�
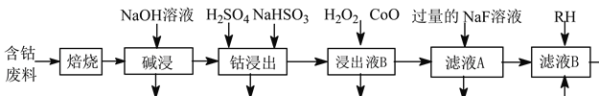
��֪���ٲ����ܾ���������ˮ
��RHΪ�л������RH���л��ܼ���ȡ����Һ�е�Ni2+
�ۼ��ֽ��������ӵ������������ʱ��PH�����ʾ��
Fe3+ | Co2+ | |
��ʼ����ʱ | 1.9 | 7.1 |
������ȫʱ | 3.7 | 9.1 |
(1)�����ա���Ŀ��_______��
(2)�������������Al2O3������Ӧ�Ļ�ѧ����ʽΪ_______��
(3) �������ữ���ܽ�����������Co3+ת��ΪCo2�������ӷ���ʽΪ_______��
(4)������ҺB���м���CoO������pHֵ��3.7~7.1��Ŀ��Ϊ_______��
(5) ����NaF��Һ�ɽ�������ת��Ϊ���������˳�ȥ����������Һ��c(F��)=1.0��10��2mol��L1������Һ��c(Ca2��)Ϊ_______mol��L1[��֪Ksp(CaF2)=1.05��10��10]��
(6) ��ȡ��ˮ���к��д�����Co2+����ˮ��������KMnO4��Һ��ֻ������Co3+��Mn2+����������ȫ��Ӧ���ĵ�n(Co2+)��n(MnO4��)=_______��