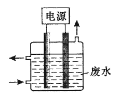
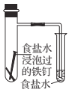
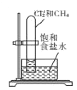
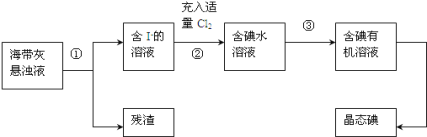
��Ŀ����
����Ŀ����ͭ���ǹ�ҵұ��ͭ��ԭ�ϣ���Ҫ�ɷ�ΪCuFeS2���Իش��������⣺
(1)��̬Cuԭ�ӵĺ�������Ų�ʽΪ________��Cu��Zn�ĵڶ������ܴ�СI2(Cu)________(�>����<������)I2(Zn)��
(2)SO2�����з��ӿռ乹��Ϊ________����SO2��Ϊ�ȵ��������������________(дһ��)��
(3)[Cr(H2O)4Br2]Br��2H2O����������λ��Ϊ_______
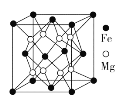
(4)��þ�Ͻ���Ŀǰ�ѷ��ֵĴ����ܶ���ߵĴ������֮һ���侧���ṹ��ͼ��ʾ����������Mgԭ�����Mg��ĿΪ____________
���𰸡�1s22s22p63s23p63d104s1��[Ar]3d104s1 > V�� ![]() 6 12
6 12
��������
(1)Cu��29��Ԫ�أ���̬Cuԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s1��[Ar]3d104s1��Zn��30��Ԫ�أ���̬Znԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s2��Cuʧȥһ�����ӱ�Ϊ��̬Cu+��������Ų�ʽΪ1s22s22p63s23p63d10��Znʧȥһ�����ӱ�Ϊ��̬Zn+��������Ų�ʽΪ1s22s22p63s23p63d104s1��Cu+�ļ۵����Ų�ʽΪ3d10��3d���ȫ��������Zn+�ȶ���Cu+����ʧ1�����ӣ���I2(Cu)��I2(Zn)���ʴ�Ϊ��1s22s22p63s23p63d104s1��[Ar]3d104s1��>��
(2)SO2��������ԭ�ӵļ۲���Ӷ���Ϊ![]() =3��������Sԭ��Ϊsp2�ӻ���������Sԭ�ӹµ��Ӷ���=
=3��������Sԭ��Ϊsp2�ӻ���������Sԭ�ӹµ��Ӷ���=![]() =1����SO2�����з��ӿռ乹��ΪV�Σ�SO2�ļ۵�����=18������SO2��Ϊ�ȵ��������������
=1����SO2�����з��ӿռ乹��ΪV�Σ�SO2�ļ۵�����=18������SO2��Ϊ�ȵ��������������![]() ���ʴ�Ϊ��V�Σ�
���ʴ�Ϊ��V�Σ�![]() ��
��
(3)[Cr(H2O)4Br2]Br��2H2O������������Ϊ4��H2O��2��Br-����λ��=4+2=6���ʴ�Ϊ��6��
(4)��ͼ��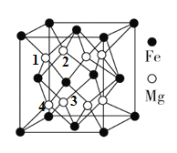 ���ԡ�1����Mgԭ��Ϊ�����þ����У��롰1����Mgԭ�Ӿ��������Mgԭ���С�2����3����4����Mgԭ�ӣ���ĿΪ3����������þ��������ϡ�ǰ3����λ��3��3=9��Mg�롰1����Mgԭ�Ӿ��������������Mgԭ�������Mgԭ����ĿΪ3+9=12�����ʴ�Ϊ��12��
���ԡ�1����Mgԭ��Ϊ�����þ����У��롰1����Mgԭ�Ӿ��������Mgԭ���С�2����3����4����Mgԭ�ӣ���ĿΪ3����������þ��������ϡ�ǰ3����λ��3��3=9��Mg�롰1����Mgԭ�Ӿ��������������Mgԭ�������Mgԭ����ĿΪ3+9=12�����ʴ�Ϊ��12��

 53���ò�ϵ�д�
53���ò�ϵ�д�����Ŀ����������������ʱ���������CO��NOx�ȶ�����Ⱦ���壬��δ�����Щ���壬�Ա����������������شش���������:
��1����֪��2NO2(g) ![]() 2NO(g)��O2(g)����H1����115.2 kJ��mol��1��
2NO(g)��O2(g)����H1����115.2 kJ��mol��1��
2O3(g) ![]() 3O2(g)����H2����286.6 kJ��mol��1��
3O2(g)����H2����286.6 kJ��mol��1��
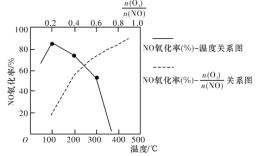
д��������NO���ò���NO2��O2���Ȼ�ѧ����ʽ��___________�������ܱ���ϵ��NO��������![]() ֵ�ı仯�Լ����¶ȵı仯������ͼ��ʾ��NO��������
ֵ�ı仯�Լ����¶ȵı仯������ͼ��ʾ��NO��������![]() ֵ������������Ҫԭ����________________________��
ֵ������������Ҫԭ����________________________��
��2��ʵ���÷�Ӧ2NO(g)+O2(g) ![]() 2NO2(g) ��H<0�ļ�ʱ��Ӧ�����������¹�ϵʽ��v��=k����c2(NO)��c(O2)��v��=k����c2(NO2)��k����k��Ϊ���ʳ��������¶�Ӱ��
2NO2(g) ��H<0�ļ�ʱ��Ӧ�����������¹�ϵʽ��v��=k����c2(NO)��c(O2)��v��=k����c2(NO2)��k����k��Ϊ���ʳ��������¶�Ӱ��
���¶�ΪT1ʱ����1L�ĺ����ܱ������У�Ͷ��0.6 molNO��0.3 molO2�ﵽƽ��ʱO2Ϊ0.2 mol���¶�ΪT2ʱ���÷�Ӧ����k��=k������T1_______ T2 (��������������С��������������)��
���о����ָ÷�Ӧ�����²�����У�
��һ����NO+NO![]() N2O2 ����ƽ�� �ڶ�����N2O2 +O2
N2O2 ����ƽ�� �ڶ�����N2O2 +O2![]() 2NO2 ����Ӧ
2NO2 ����Ӧ
���пɽ�����Ϊ�ڶ�����Ӧ��Ӱ���һ����ƽ�⣬��һ����Ӧ�У�v1��=k1����c2(NO)��v1��=k1����c(N2O2)
����������ȷ����______
A.ͬһ�¶��£�ƽ��ʱ��һ����Ӧ��![]() Խ��Ӧ����̶�Խ��
Խ��Ӧ����̶�Խ��
B.�ڶ�����Ӧ���ʵͣ����ת����Ҳ��
C.�ڶ����Ļ�ܱȵ�һ���Ļ�ܵ�
D.������Ӧ�������ɵڶ�����Ӧ���ʾ���
��3����ѧ���о�����һ�ָ�Ч���������Խ�CO��NO2����ת��Ϊ����Ⱦ���壬��Ӧ����ʽΪ��2NO2(g)+4CO(g) ![]() 4CO2(g)+N2(g) ��H<0
4CO2(g)+N2(g) ��H<0
ij�¶��£���10L�ܱ������зֱ����0.1molNO2��0.2 molCO������������Ӧ�����ŷ�Ӧ�Ľ��У������ڵ�ѹǿ�仯���±���ʾ��
ʱ��/min | 0 | 2 | 4 | 6 | 8 | 10 | 12 |
ѹǿ/kPa | 75 | 73.4 | 71.95 | 70.7 | 69.7 | 68.75 | 68.75 |
�ش��������⣺
���ڴ��¶��£���Ӧ��ƽ�ⳣ��Kp=_________kPa-1(KpΪ�Է�ѹ��ʾ��ƽ�ⳣ������������ȷ��С�����2λ)���������¶Ȳ��䣬�ٽ�CO��CO2����Ũ�ȷֱ�����һ������ƽ��_____(���������������������������ƶ���)��
�������¶Ƚ��ͣ��ٴ�ƽ�����ԭƽ�������ϵѹǿ(p��)��α仯��_______(����������������С������������)��ԭ����_____________________��
����Ŀ������ʵ�鷽���ܴﵽʵ��������( )
��� | A | B | C | D |
ʵ�鷽�� |
|
Ƭ�̺��� |
|
���ڹ����� |
ʵ���� | �����������ⷴӦ |
| ����������ɫ��� ����������ɫ��dz | ���������������ӳɷ�Ӧ |
A.AB.BC.CD.D
����Ŀ����һ���¶��£�10mL 0.40mol/L H2O2 ��Һ�������ֽ⡣��ͬʱ�̲������O2�����(������Ϊ��״��)���±���
t/min | 0 | 2 | 4 | 6 | 8 | 10 |
V(O2)/mL | 0.0 | 9.9 | 17.2 | 22.4 | 26.5 | 29.9 |
������������ȷ����(��Һ����仯���Բ���)
A.0~6min��ƽ����Ӧ���ʣ���(H2O2)=3.3��10-2mol/(L��min)
B.6~10min��ƽ����Ӧ���ʣ���(H2O2)��3.3��10-2mol/(L��min)
C.��Ӧ��6minʱ��c(H2O2)=0.30mol/L
D.��Ӧ��6minʱ��H2O2 �ֽ���50%