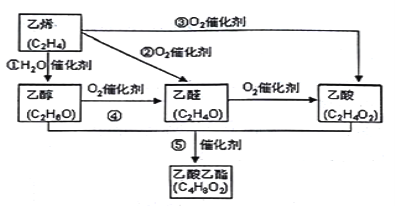
МвДҝДЪИЭ
ЎҫМвДҝЎҝоЬКЗИЛМеұШРиөДОўБҝФӘЛШЈ¬ә¬оЬ»ҜәПОпЧчОӘСХБПЈ¬ҫЯУРУЖҫГөДАъК·Ј¬ФЪ»ъРөЦЖФмЎўҙЕРФІДБПөИБмУтТІҫЯУР№г·әөДУҰУГЈ¬Зл»ШҙрПВБРОКМв:
ЈЁ1Ј©Co»щМ¬ФӯЧУөДөзЧУЕЕІјКҪОӘ__________________________Ј»
ЈЁ2Ј©МӘЭјоЬҪьДкАҙФЪ№вөзІДБПЎў·ЗПЯРФ№вС§ІДБПЎў№в¶ҜБҰС§ЦРөД№вГфјБЎўҙЯ»ҜјБөИ·ҪГжөГөҪ№г·әөДУҰУГЈ¬ЖдҪб№№ИзНјЛщКҫЈ¬ЦРРДАлЧУОӘоЬАлЧУЎЈ
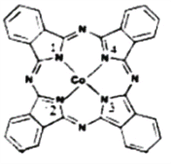
ўЩМӘЭјоЬЦРИэЦЦ·ЗҪрКфФӯЧУөДөзёәРФУРҙуөҪРЎөДЛіРтОӘ____________Ј¬(УГПаУҰөДФӘЛШ·ыәЕЧчҙр)Ј»МјФӯЧУөДФУ»Ҝ№мөААаРНОӘ___________________________Ј»
ўЪУлоЬАлЧУНЁ№эЕдО»ҪЎҪбәПөДөӘФӯЧУөДұаәЕКЗ___________________________Ј»
ЈЁ3Ј©УГKCNҙҰАнә¬Co2+өДСОИЬТәЈ¬УРәмЙ«өДCo(CN)2ОціцЈ¬Ҫ«ЛьИЬУЪ№эБҝөДKCNИЬТәәуЈ¬ҝЙЙъіЙЧПЙ«өД[Co(CN)6]4-Ј¬ёГЕдАлЧУЦРөДЕдО»МеОӘ________Ј¬ЕдО»ФӯЧУОӘ____________________Ј»
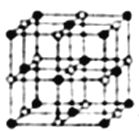
ЈЁ4Ј©CoөДТ»ЦЦСх»ҜОпөДҫ§°ыИзНјЛщКҫЈ¬ФЪёГҫ§МеЦРУлТ»ёцоЬФӯЧУөИҫаАлЗТЧоҪьөДоЬФӯЧУУР_____ёцЈ»УлТ»ёцоЬФӯЧУөИҫаАлЗТҙОҪьөДСхФӯЧУУР______ёцЈ»ИфёГоЬөДСх»ҜОпҫ§МеЦРоЬФӯЧУУлёъЛьЧоҪьБЪөДСхФӯЧУЦ®јдөДҫаАлОӘrЈ¬ёГоЬФӯЧУУлёъЛьҙОҪьБЪөДСхФӯЧУЦ®јдөДҫаАлОӘ______Ј»ТСЦӘФЪёГоЬөДСх»ҜОпҫ§МеЦРоЬФӯЧУөД°лҫ¶ОӘapmЈ¬СхФӯЧУөД°лҫ¶ОӘbpmЈ¬ЛьГЗФЪҫ§МеЦРКЗҪфГЬҪУҙҘөДЈ¬ФтФЪёГоЬөДСх»ҜОпҫ§МеЦРФӯЧУөДҝХјдАыУГВКОӘ____(УГә¬aЎўbөДКҪЧУұнКҫ)ЎЈ
ЈЁ5Ј©ЦюІЁІДБПҝЖС§№ъјТКөСйКТТ»ёцҝЖСРРЎЧй·ўПЦБЛФЪ5KПВіКПЦі¬өјРФөДҫ§МеЈ¬ёГҫ§МеҫЯУРCoO2өДІгЧҙҪб№№(ИзПВНјЛщКҫЈ¬РЎЗтұнКҫCoФӯЧУЈ¬ҙуЗтұнКҫOФӯЧУ)ЎЈПВБРУГҙЦПЯ»ӯіцөДЦШёҙҪб№№өҘФӘКҫТвНјІ»ДЬГиКцCoO2өД»ҜС§ЧйіЙөДКЗ_______ЎЈ

Ўҫҙр°ёЎҝ 1s22s22p63s23p63d74s2 N>C>H sp2 2Ј¬4 CN- N 12 8 ![]() r 2ҰР/3ЎБ(a2+b2)/(a+b)3 D
r 2ҰР/3ЎБ(a2+b2)/(a+b)3 D
ЎҫҪвОцЎҝЈЁ1Ј©CoОӘ27әЕФӘЛШЈ¬әЛНвөзЧУКэОӘ27Ј¬ёщҫЭДЬБҝЧоөНФӯАнЈ¬ЖдәЛНвөзЧУЕЕІјКҪОӘЈә1s22s22p63s23p63d74s2 Ј»ХэИ·ҙр°ёЈә 1s22s22p63s23p63d74s2ЎЈ
ЈЁ2Ј©ўЩМӘЭјоЬЦРИэЦЦ·ЗҪрКфФӯЧУОӘCЎўNЎўHЈ¬Н¬ЦЬЖЪҙУЧуөҪУТөзёәРФФцҙуЈ¬·ЗҪрКфРФФҪЗҝЈ¬ЛщТФөзёәРФN>C>HЈ»·ЦЧУЦРМјФӯЧУҫщРОіЙ3ёцҰТјьЈ¬Г»УР№В¶ФөзЧУЈ¬ФУ»Ҝ№мөАКэОӘ3Ј¬МјФӯЧУөДФУ»Ҝ№мөАОӘsp2ФУ»ҜЈ»ХэИ·ҙр°ёЈәN>C>H Ј» sp2ЎЈ
ўЪә¬УР№ВөзЧУ¶ФNУлCoНЁ№эЕдО»јьҪбәПРОіЙЕдО»јьәуЈ¬РОіЙ4¶Ф№ІУГөзЧУ¶ФЈ¬1әЕЎў3әЕNФӯЧУРОіЙ3¶Ф№ІУГөзЧУ¶ФОӘЖХНЁөД№ІјЫјьЈ»2әЕЎў4әЕNФӯЧУРОіЙ4¶Ф№ІУГөзЧУ¶ФЈ¬УлCoФӯЧУНЁ№эЕдО»јьҪбәПЈ»ХэИ·ҙр°ёЈә2Ј¬4ЎЈ
ЈЁ3Ј©НЁ№эМвТвҝЙЦӘЈ¬ёГАлЧУЦРөДЕдО»МеОӘCN-Ј¬РОіЙЕдО»јьөДФӯЧУОӘNЈ»ХэИ·ҙр°ёЈәCN-Ј»NЎЈ
ЈЁ4Ј©јЩЙиәЪЙ«ЗтКЗоЬФӯЧУЈ¬ТФ¶ҘөгоЬФӯЧУОӘСРҫҝ¶ФПуЈ¬УлЦ®ЧоҪьөДоЬФӯЧУО»УЪГжРДЈ¬Гҝёц¶Ҙөг8ёцҫ§°ы№ІУГЈ¬ГҝёцГжРДОӘ2ёцҫ§°ы№ІУГЈ¬ФЪёГҫ§°ыЦРУлТ»ёцоЬФӯЧУөИҫаАлЗТЧоҪьөДоЬФӯЧУөДёцКэОӘ3ЎБ8/2=12Ј»УлТ»ёцоЬФӯЧУөИҫаАлЗТҙОҪьөДСхФӯЧУУР8ёцЈ»оЬАлЧУУлёъЛьҙОҪьБЪөДСхАлЧУЦ®јдҫаАлОӘҫ§°ыМе¶ФҪЗПЯөДТ»°лЈ¬ҫаАлОӘ ![]() rЈ»оЬөДСх»ҜОпҫ§МеЦР№Іә¬УРоЬФӯЧУ8ЎБ1/8+6ЎБ1/2=4ёцЈ¬ә¬УРСхФӯЧУ12ЎБ1/4+1=4Ј¬јҙҫ§°ыЦРә¬УР4ёцCoOЈ¬Ме»эОӘ4/3ҰРЎБЈЁa2+b2)ЎБ4Ј¬ҫ§°ыөДұЯіӨОӘ(2a+2b)Ј¬ҫ§°ыөДМе»эОӘ(2a+2b)3Ј¬оЬөДСх»ҜОпҫ§МеЦРФӯЧУөДҝХјдАыУГВКОӘ[4/3ҰРЎБЈЁa2+b2)ЎБ4]/(2a+2b)3=2ҰР/3ЎБ(a2+b2)/(a+b)3Ј»ХэИ·ҙр°ёЈә12Ј» 8 Ј»
rЈ»оЬөДСх»ҜОпҫ§МеЦР№Іә¬УРоЬФӯЧУ8ЎБ1/8+6ЎБ1/2=4ёцЈ¬ә¬УРСхФӯЧУ12ЎБ1/4+1=4Ј¬јҙҫ§°ыЦРә¬УР4ёцCoOЈ¬Ме»эОӘ4/3ҰРЎБЈЁa2+b2)ЎБ4Ј¬ҫ§°ыөДұЯіӨОӘ(2a+2b)Ј¬ҫ§°ыөДМе»эОӘ(2a+2b)3Ј¬оЬөДСх»ҜОпҫ§МеЦРФӯЧУөДҝХјдАыУГВКОӘ[4/3ҰРЎБЈЁa2+b2)ЎБ4]/(2a+2b)3=2ҰР/3ЎБ(a2+b2)/(a+b)3Ј»ХэИ·ҙр°ёЈә12Ј» 8 Ј» ![]() r Ј»(2ҰР/3ЎБ(a2+b2)/(a+b)3ЎЈ
r Ј»(2ҰР/3ЎБ(a2+b2)/(a+b)3ЎЈ
ЈЁ5Ј©CoO2ЦШёҙҪб№№өҘФӘЦРоЬФӯЧУЈәСхФӯЧУКэДҝЦ®ұИУГОӘ1:2Ј»УРТФПВНјПсҝЙЦӘЈәAЦРоЬФӯЧУЈәСхФӯЧУ=1:ЈЁ4ЎБ1/2Ј©=1:2Ј¬·ыәПЈ»BЦРоЬФӯЧУЈәСхФӯЧУ=ЈЁ1+4ЎБ1/4Ј©:4=1:2Ј¬·ыәПЈ»CЦРоЬФӯЧУЈәСхФӯЧУ=ЈЁ4ЎБ1/4Ј©:ЈЁ4ЎБ1/2Ј©=1:2Ј¬·ыәПЈ»DЦРЦРоЬФӯЧУЈәСхФӯЧУ=1ЈәЈЁ4ЎБ1/4Ј©=1:1Ј¬І»·ыәПЈ»ХэИ·СЎПоDЎЈ

ЎҫМвДҝЎҝнЪ(Te)ОӘөЪVIAФӘЛШЈ¬ЖдөҘЦКЖҫҪиУЕБјөДРФДЬіЙОӘЦЖЧчәПҪрМнјУјБЎў°лөјМеЎў№вөзФӘјюөДЦчМеІДБПЈ¬Іўұ»№г·әУҰУГУЪТұҪрЎўәҪҝХәҪМмЎўөзЧУөИБмУтЎЈҝЙҙУҫ«Б¶НӯөДСфј«Да(ЦчТӘіЙ·ЦОӘCu2Te)ЦР»ШКХнЪЈ¬
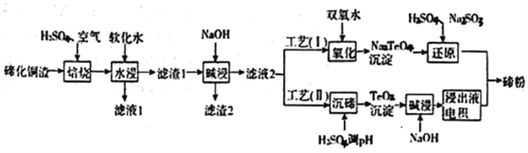
ЈЁ1Ј©Ў°ЕаЙХЎұәу,И·ЦчТӘТФTeO2РОКҪҙжФЪЈ¬РҙіцПаУҰ·ҙУҰөДАлЧУ·ҪіМКҪ:________________________ЎЈ
ЈЁ2Ј©ОӘБЛСЎФсЧојСөДЕаЙХ№ӨТХҪшРРБЛОВ¶ИәНБтЛбјУИлБҝөДМхјюКФСйЈ¬Ҫб№ыИзПВұнЛщКҫ:
ОВ¶И/Ўж | БтЛбјУИлБҝ(АнВЫБҝұ¶Кэ) | ҪюіцВК/% | |
Cu | Te | ||
450 | 1.25 | 77.3 | 2.63 |
460 | 1.00 | 80.29 | 2.81 |
1.25 | 89.86 | 2.87 | |
1.50 | 92.31 | 7.70 | |
500 | 1.25 | 59.83 | 5.48 |
550 | 1.25 | 11.65 | 10.63 |
ФтКөСйЦРУҰСЎФсөДМхјюОӘ_________________Ј¬ФӯТтОӘ______________________________ЎЈ
ЈЁ3Ј©ВЛФь1ФЪјоҪюКұ·ўЙъөД»ҜС§·ҪіМКҪОӘ_____________________________ЎЈ
ЈЁ4Ј©№ӨТХЈЁIЈ©ЦРЈ¬Ў°»№ФӯЎұКұ·ўЙъөДЧЬөД»ҜС§·ҪіМКҪОӘ____________________________ЎЈ
ЈЁ5Ј©УЙУЪ№ӨТХЈЁIЈ©ЦРЎ°Сх»ҜЎұ¶ФИЬТәәНОпБПМхјюТӘЗуёЯЎЈУРСРҫҝХЯІЙУГ№ӨТХЈЁIIЈ©»сөГ°х.ФтЎ°өз»эЎұ№эіМЦРЈ¬Тхј«өДөзј«·ҙУҰКҪОӘ____________________________________ЎЈ
ЈЁ6Ј©№ӨТөЙъІъЦРЈ¬ВЛФь2ҫӯБтЛбЛбҪюәуөГВЛТә3әНВЛФь3ЎЈ
ўЩВЛТә3УлВЛТә1әПҫ®ЎЈҪшИлНӯөз»эПөНіЎЈёГҙҰАнҙлК©өДУЕөгОӘ_____________________________ЎЈ
ўЪВЛФь3ЦРИфә¬Auspan>әНAgЈ¬ҝЙУГ_____Ҫ«¶юХЯ·ЦАлЎЈ(МоЧЦДё)
A.НхЛ® B.ПЎПхЛб C.ЕЁЗвСх»ҜДЖИЬТә D.ЕЁСОЛб
ЎҫМвДҝЎҝAЎўBЎўCЎўDЎўEКЗОеЦЦ¶МЦЬЖЪФӘЛШЎЈТСЦӘЈәЛьГЗөДФӯЧУРтКэТАҙОФцҙуЈ¬AКЗФӘЛШЦЬЖЪұнЦРФӯЧУ°лҫ¶ЧоРЎөДФӘЛШЈ»BФӯЧУЧоНвІгөзЧУКэұИЖдҙОНвІгөзЧУКэ¶а2Ј¬CКЗEөДБЪЧеФӘЛШЈ»DәНEөДФӯЧУРтКэЦ®әНОӘ30Ј¬ЗТDөДЧеРтКэУлЦЬЖЪКэПаөИЎЈјЧЎўТТЎўұыЎў¶ЎКЗЛьГЗБҪБҪРОіЙөД»ҜәПОпЈ¬ЖдЦРјЧ·ЦЧУЦРә¬УР18ёцөзЧУЎЈ
ОпЦКЧйіЙ | јЧ | ТТ | ұы | ¶Ў |
»ҜәПОпЦРёчФӘЛШ ФӯЧУёцКэұИ | AәНC 1:1 | BәНA 1:4 | DәНE 1:3 | BәНE 1:4 |
Зл»ШҙрПВБРОКМвЈә
ЈЁ1Ј©ФӘЛШEФЪЦЬЖЪұнЦРөДО»ЦГОӘ___________________________Ј»
ЈЁ2Ј©°СDөДөҘЦК·ЕөҪNaOHИЬТәЦРЈ¬·ҙУҰөД»ҜС§·ҪіМКҪОӘЈә_______________________Ј»
ЈЁ3Ј©УГөзЧУКҪұнКҫјЧөДРОіЙ№эіМЈә_________________________Ј»
ЈЁ4Ј©ФЪГЬұХИЭЖчЦРідИлBC2ЎўBCәНТТөД»мәПЖшМе№ІmgЈ¬ИфјУИлЧгБҝNa2O2,ід·ЦХсөҙІўІ»¶ПУГөз»р»ЁөгИјЦБ·ҙУҰНкИ«Ј¬ІвөГ№ММеЦКБҝФцЦШmgЈ¬ФтBC2УлТТөДМе»эұИОӘ________________Ј»
ЈЁ5Ј©УР200mL MgCl2әНұыөД»мәПИЬТәЈ¬ЖдЦРc(Mg2+)ЈҪ 0.2 molЎӨ L-1Ј¬c(Cl-)ЈҪ 1.3molЎӨL-1Ј¬ТӘК№Mg2Ј«И«ІҝЧӘ»ҜОӘіБөн·ЦАліцАҙЈ¬ЦБЙЩРиТӘ4 molЎӨL-1 NaOH ИЬТәөДМе»эКЗЈә______ЎЈ