��Ŀ����
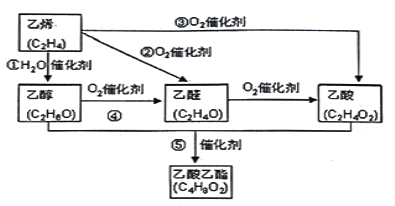
����Ŀ����:��ϩ�����Ǻ���һ������ʯ�ͻ���ˮƽ����Ҫ��־����ͼ������ϩ�ϳ������������ܵĺϳ�·�ߣ�
��ش��������⣺
��1����Ӧ�ܵĻ�ѧ����ʽΪ_____________________________ ��
��2����ʵ�����Ʊ���������ʱ���õ����͵�̼������Һ���������ǣ�___________________��
��3���Ҵ��ĽṹʽΪ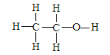 ����ʵ��֤���Ҵ�������һ��������ԭ�ӵķ�Ӧ�Ļ�ѧ����ʽΪ _________________________________��
����ʵ��֤���Ҵ�������һ��������ԭ�ӵķ�Ӧ�Ļ�ѧ����ʽΪ _________________________________��
�������к������ᣬ�����ڳ��³�ѹ����һ����ɫ��ճ�Ⱥܴ��Һ�塣ȡ9.0g������������Na��Ӧ���ڱ�״���¿��ռ���2.24L���壻��ȡ9.0g������������NaHCO3��Һ��Ӧ�����ɵ�CO2�����ڱ�״�������Ϊ2.24L����֪��������к���һ��������ش��������⣺
��1���������Է�������Ϊ��_______________________��
��2����Ũ������ڵ������£��������������Ӧ���ɻ�״��������д���÷�Ӧ�Ļ�ѧ��Ӧ����ʽ��________________________���䷴Ӧ����Ϊ��_______________��
���𰸡�2CH3CH2OH + O2![]() 2CH3CHO + 2H2O���ջӷ�������������Ҵ������������������ܽ��2CH3CH2OH + 2Na �� 2CH3CH2ONa + H2��902CH3CH��OH��COOH
2CH3CHO + 2H2O���ջӷ�������������Ҵ������������������ܽ��2CH3CH2OH + 2Na �� 2CH3CH2ONa + H2��902CH3CH��OH��COOH![]()
 +2H2Oȡ����Ӧ��������Ӧ
+2H2Oȡ����Ӧ��������Ӧ
��������
����ͼ��֪����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ����Ҵ�����������ȩ����ȩ�����������ᣬ����ϩ������������ȩ����ϩ�������������ᣬ�Ҵ������ᷢ��������Ӧ��������������
��1����Ӧ��Ϊ�Ҵ�������������ȩ����ѧ����ʽΪ2CH3CH2OH + O2![]() 2CH3CHO + 2H2O���ʴ�Ϊ��2CH3CH2OH + O2
2CH3CHO + 2H2O���ʴ�Ϊ��2CH3CH2OH + O2![]() 2CH3CHO + 2H2O��
2CH3CHO + 2H2O��
��2����ʵ�����Ʊ���������ʱ�����͵�̼������Һ�������ջӷ�������������Ҵ������������������ܽ�ȣ��ʴ�Ϊ�����ջӷ�������������Ҵ������������������ܽ�ȣ�
��3���Ҵ��ĽṹʽΪ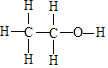 ����ͬ���칹��ĽṹʽΪ
����ͬ���칹��ĽṹʽΪ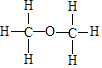 ������ͨ��������Ƶķ�Ӧ֤���Ҵ�������һ��������ԭ�ӣ���Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH+2Na��2CH3CH2ONa+H2�����ʴ�Ϊ��2CH3CH2OH+2Na��2CH3CH2ONa+H2����
������ͨ��������Ƶķ�Ӧ֤���Ҵ�������һ��������ԭ�ӣ���Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH+2Na��2CH3CH2ONa+H2�����ʴ�Ϊ��2CH3CH2OH+2Na��2CH3CH2ONa+H2����
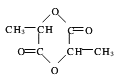
������1��9.0g����������Ľ����Ʒ�Ӧ��������ɵ��������Ϊ2.24L����������ѻ���Ϊ��״���������������������к��й������Ǵ��ǻ����Ȼ����������������ʵ���Ϊ![]() =0.1mol�������2-OH��-COOH����H2��֪��9.0g���Ậ�������ǻ������Ȼ���0.2mol�����ǻ����Ȼ���0.1mol����ȡ9.0g����������ı���NaHCO3��Һ��Ӧ��˵����������л������Ȼ������ɶ�����̼�����ʵ���Ϊ0.1mol������-COOH��CO2��֪��9.0g���Ậ0.1mol-COOH������9.0g�����л�����0.1mol-OH����90g��������к�1mol-OH��1mol-COOH����֪��������л���һ��-CH3����90-17-45-15=13����������ӳ���-OH��-COOH��-CH3�⣬����-CH�ṹ������Ľṹ��ʽΪCH3CH��OH��COOH���������Է�������Ϊ��12��3+1��6+16��3=90���ʴ�Ϊ��90��
=0.1mol�������2-OH��-COOH����H2��֪��9.0g���Ậ�������ǻ������Ȼ���0.2mol�����ǻ����Ȼ���0.1mol����ȡ9.0g����������ı���NaHCO3��Һ��Ӧ��˵����������л������Ȼ������ɶ�����̼�����ʵ���Ϊ0.1mol������-COOH��CO2��֪��9.0g���Ậ0.1mol-COOH������9.0g�����л�����0.1mol-OH����90g��������к�1mol-OH��1mol-COOH����֪��������л���һ��-CH3����90-17-45-15=13����������ӳ���-OH��-COOH��-CH3�⣬����-CH�ṹ������Ľṹ��ʽΪCH3CH��OH��COOH���������Է�������Ϊ��12��3+1��6+16��3=90���ʴ�Ϊ��90��
��2������Ľṹ��ʽΪCH3CH��OH��COOH��2�����������һ�������¿ɷ���������Ӧ������Ԫ��״�������Ӧ����ʽΪ��2CH3CH��OH��COOH![]()
 +2H2O���ʴ�Ϊ��2CH3CH��OH��COOH
+2H2O���ʴ�Ϊ��2CH3CH��OH��COOH![]()
 +2H2O��������Ӧ����ȡ����Ӧ����
+2H2O��������Ӧ����ȡ����Ӧ����

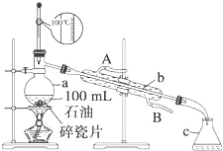
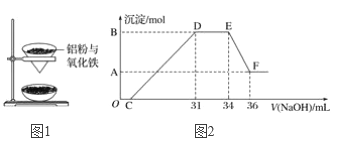
����Ŀ����ͼ1��ijͬѧ��ʵ�����н������ȷ�Ӧ(������)��ʵ��װ�ã�ʵ�������۲쵽������֮һΪ��ֽ©�����²����մ���������������ɳ������
��1��д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________________________��
��2��Ϊ�������ȷ�Ӧ��Ĺ���ɷ֣�����д�±���
����ɷ� | �������� | ʵ����������� |
�� | ____________ | ___________ |
������ | _____________ | ____________ |
��3��̽�����ȷ�Ӧ���������ʣ�����Ӧ�������еĹ�����������ձ��У�����һ����ϡ���ᣬ������ȫ�ܽ⣬��Ӧ������������ų�(���ý����ɰ�ϡHNO3)��ԭΪNH4NO3)���ڷ�Ӧ�����Һ�л����μ�4 mol��L��1��NaOH��Һ���������������ʵ���(mol)�����NaOH��Һ�����(ml)�Ĺ�ϵ��ͼ2��ʾ��
��1д��DE�η�����Ӧ�����ӷ���ʽ��_____________________________________________��
��B��A�IJ�ֵΪ_______________________________________________________________��