��Ŀ����
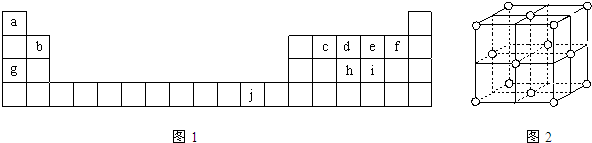
 Ԫ�����ڱ��е������ڵĽ���Ԫ���������Ϳ������зdz���Ҫ��ʹ�ü�ֵ��
Ԫ�����ڱ��е������ڵĽ���Ԫ���������Ϳ������зdz���Ҫ��ʹ�ü�ֵ����1���ⶨ���������ĺ���ʱ���Ƚ���������ԭΪ���������ٲ����ڷ���������ɫ�����ñ�ɫ���ⶨ���������к��и�������ʱ��Բⶨ�и��ţ���صķ�Ӧ���£�
4FeCl3+2NH2OH?HCl��4FeCl2+N2O��+6HCl+H2O
��Fe2+�ڻ�̬ʱ����������Ų�ʽ
���ǰ��У�NH2OH������SP3�ӻ���ԭ����
��Fe2+���ڷ������γɵ�������У���λ��Ϊ
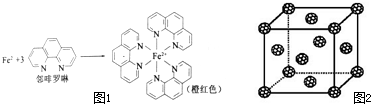
��2��������ͭ��Һ�м��������ˮ��Ȼ����������Ҵ�����Һ����������ɫ��[Cu��NH3��4]SO4���壬�þ����к��еĻ�ѧ��������
��3��������ͭ��Һ�м��������ˮ��������[Cu��NH3��4]2+����֪NF3��NH3�Ŀռ乹�Ͷ��������Σ���NF3 ������Cu2+�γ������ӣ���ԭ����
��4�������Ni��CO��4�����³�Һ̬��������CCl4�������л��ܼ�����̬Ni��CO��4����
��5������Ѿ����������������������Χ�ɵĿ�϶�����������϶�������� �ڵ縺����С��ԭ�ӿ���Ϊ�������C60 ����Ŀ�϶�У��γɾ������õij����ԵIJ���C60 ������ְ�C60������ʵ㣬�þ���ľ����ṹ��ͼ2��ʾ����ÿ���������϶����һ��ԭ�ӣ���ȫ������C60 ������������϶�����γɵIJ���C60 ������Ļ�ѧʽΪ
��������1��������26��Ԫ�أ���ԭ�Ӻ�����26�����ӣ���ԭ��ʧȥ2�����ӱ���������ӣ����ݹ���ԭ��д���������Ӻ�������Ų�ʽ��
�ڸ��ݼ۲���ӶԻ�������ȷ���ǰ�NH2OH�д���sp3�ӻ���ԭ�ӣ�
����λ����������λ��������һ���γ���ɼ�����λԭ�ӵ�������
��2��Cu2+�ṩ�չ����Nԭ���ṩ�¶Ե��ӣ��γ���λ����N��Hԭ��֮���Թ��ۼ���ϣ��Ƚ�������������������Ӽ���ϣ�
��3�����ݵ縺�ԵĽǶȷ�����
��4��Ni��CO��4������ΪҺ̬��������CCl4�������л��ܼ������ڷ��Ӿ��壻
��5�����������У��ڳ��������Σ�Ȼ������ŵ��ĸ�������Χ�ɵĿ�϶���ͽ����������϶����ͼ��ʾ��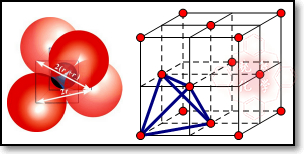 �����������ṹ�У�ÿ��С�������Ӧ1���������϶��һ����8�����������϶����ͼ����������һ�����������ڵ縺����С��ԭ����K�����Ծ�����һ����8��kԭ�ӣ�1+6��
�����������ṹ�У�ÿ��С�������Ӧ1���������϶��һ����8�����������϶����ͼ����������һ�����������ڵ縺����С��ԭ����K�����Ծ�����һ����8��kԭ�ӣ�1+6��
=4��C60���ӣ�Ȼ�������ѧʽ��
�ڸ��ݼ۲���ӶԻ�������ȷ���ǰ�NH2OH�д���sp3�ӻ���ԭ�ӣ�
����λ����������λ��������һ���γ���ɼ�����λԭ�ӵ�������
��2��Cu2+�ṩ�չ����Nԭ���ṩ�¶Ե��ӣ��γ���λ����N��Hԭ��֮���Թ��ۼ���ϣ��Ƚ�������������������Ӽ���ϣ�
��3�����ݵ縺�ԵĽǶȷ�����
��4��Ni��CO��4������ΪҺ̬��������CCl4�������л��ܼ������ڷ��Ӿ��壻
��5�����������У��ڳ��������Σ�Ȼ������ŵ��ĸ�������Χ�ɵĿ�϶���ͽ����������϶����ͼ��ʾ��
 �����������ṹ�У�ÿ��С�������Ӧ1���������϶��һ����8�����������϶����ͼ����������һ�����������ڵ縺����С��ԭ����K�����Ծ�����һ����8��kԭ�ӣ�1+6��
�����������ṹ�У�ÿ��С�������Ӧ1���������϶��һ����8�����������϶����ͼ����������һ�����������ڵ縺����С��ԭ����K�����Ծ�����һ����8��kԭ�ӣ�1+6��| 1 |
| 2 |
����⣺��1��������26��Ԫ�أ���ԭ�Ӻ�����26�����ӣ���ԭ��ʧȥ2�����ӱ���������ӣ�Fe2+�ڻ�̬ʱ����������Ų�ʽΪls22s22p63s23p63d6��[Ar]3d6��
�ʴ�Ϊ��ls22s22p63s23p63d6��[Ar]3d6��
���ǰ������У��۲���Ӷ�Ϊ4��ԭ����N��Oԭ�ӣ����Բ���sp3�ӻ���ԭ����N��Oԭ�ӣ��ʴ�Ϊ��N��O��
����λ����������λ��������һ���γ���ɼ�����λԭ�ӵ�����������Fe2+���ڷ������γɵ������γɹ�����ͼ1���У���λ��Ϊ6���ʴ�Ϊ��6��
��2��Cu2+�ṩ�չ����Nԭ���ṩ�¶Ե��ӣ�Cu2+��NH3����֮���γ���λ����NH3������N��Hԭ��֮���Թ��ۼ���ϣ��Ƚ�����[Cu��NH3��4]2+���������SO42-�����Ӽ���ϣ�
�ʴ�Ϊ�����Ӽ������ۼ�����λ����
��3��N��F��H����Ԫ�صĵ縺�ԣ�F��N��H������NH3�й��õ��Ӷ�ƫ��N������NF3�У����õ��Ӷ�ƫ��F��ƫ��Nԭ�ӣ�
�ʴ�Ϊ��F�ĵ縺�Ա�N��N-F�ɼ����Ӷ���Fƫ�ƣ�����NF3��Nԭ�Ӻ˶���¶Ե��ӵ�����������ǿ�������γ���λ����NF3������Cu2+�γ������ӣ�
��4��Ni��CO��4������ΪҺ̬��������CCl4�������л��ܼ�����̬Ni��CO��4���ڷ��Ӿ��壬�ʴ�Ϊ�����ӣ�
��5�����������У��ڳ��������Σ�Ȼ������ŵ��ĸ�������Χ�ɵĿ�϶���ͽ����������϶����ͼ��ʾ�� �����������ṹ�У�ÿ��С�������Ӧ1���������϶��һ����8�����������϶����ͼ����������һ�����������ڵ縺����С��ԭ����K�����Ծ�����һ����8��Kԭ�ӣ�1+6��
�����������ṹ�У�ÿ��С�������Ӧ1���������϶��һ����8�����������϶����ͼ����������һ�����������ڵ縺����С��ԭ����K�����Ծ�����һ����8��Kԭ�ӣ�1+6��
=4��C60���ӣ������ K8��C60��4����ѧʽΪK2C60���ʴ�Ϊ��K2C60��
�ʴ�Ϊ��ls22s22p63s23p63d6��[Ar]3d6��
���ǰ������У��۲���Ӷ�Ϊ4��ԭ����N��Oԭ�ӣ����Բ���sp3�ӻ���ԭ����N��Oԭ�ӣ��ʴ�Ϊ��N��O��
����λ����������λ��������һ���γ���ɼ�����λԭ�ӵ�����������Fe2+���ڷ������γɵ������γɹ�����ͼ1���У���λ��Ϊ6���ʴ�Ϊ��6��
��2��Cu2+�ṩ�չ����Nԭ���ṩ�¶Ե��ӣ�Cu2+��NH3����֮���γ���λ����NH3������N��Hԭ��֮���Թ��ۼ���ϣ��Ƚ�����[Cu��NH3��4]2+���������SO42-�����Ӽ���ϣ�
�ʴ�Ϊ�����Ӽ������ۼ�����λ����
��3��N��F��H����Ԫ�صĵ縺�ԣ�F��N��H������NH3�й��õ��Ӷ�ƫ��N������NF3�У����õ��Ӷ�ƫ��F��ƫ��Nԭ�ӣ�
�ʴ�Ϊ��F�ĵ縺�Ա�N��N-F�ɼ����Ӷ���Fƫ�ƣ�����NF3��Nԭ�Ӻ˶���¶Ե��ӵ�����������ǿ�������γ���λ����NF3������Cu2+�γ������ӣ�
��4��Ni��CO��4������ΪҺ̬��������CCl4�������л��ܼ�����̬Ni��CO��4���ڷ��Ӿ��壬�ʴ�Ϊ�����ӣ�
��5�����������У��ڳ��������Σ�Ȼ������ŵ��ĸ�������Χ�ɵĿ�϶���ͽ����������϶����ͼ��ʾ��
 �����������ṹ�У�ÿ��С�������Ӧ1���������϶��һ����8�����������϶����ͼ����������һ�����������ڵ縺����С��ԭ����K�����Ծ�����һ����8��Kԭ�ӣ�1+6��
�����������ṹ�У�ÿ��С�������Ӧ1���������϶��һ����8�����������϶����ͼ����������һ�����������ڵ縺����С��ԭ����K�����Ծ�����һ����8��Kԭ�ӣ�1+6��| 1 |
| 2 |
����������Ŀ�ۺ��Խϴ��漰���塢��ѧ�����ӻ�������縺�ԡ���������ȣ����������ѶȽϴ�Ϊ�״��㣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
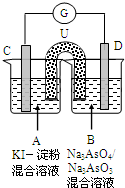 ��ͼ��һ�绯ѧʵ��װ�ã�ͼ��C��D��Ϊ���缫��UΪ���ţ�G�����������ƣ���ָ������ƫ���Դ������
��ͼ��һ�绯ѧʵ��װ�ã�ͼ��C��D��Ϊ���缫��UΪ���ţ�G�����������ƣ���ָ������ƫ���Դ������